Non-canonical PD-1 signaling in cancer and its potential implications in clinic
- PMID: 33593825
- PMCID: PMC7888367
- DOI: 10.1136/jitc-2020-001230
Non-canonical PD-1 signaling in cancer and its potential implications in clinic
Abstract
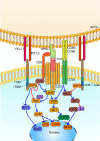
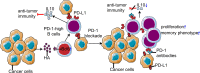
Programmed cell death 1 (PD-1)-based immunotherapy has revolutionized the treatment of various cancers. However, only a certain group of patients benefit from PD-1 blockade therapy and many patients succumb to hyperprogressive disease. Although, CD8 T cells and conventional T cells are generally considered to be the primary source of PD-1 in cancer, accumulating evidence suggests that other distinct cell types, including B cells, regulatory T cells, natural killer cells, dendritic cells, tumor-associated macrophages and cancer cells, also express PD-1. Hence, the response of patients with cancer to PD-1 blockade therapy is a cumulative effect of anti-PD-1 antibodies acting on a myriad of cell types. Although, the contribution of CD8 T cells to PD-1 blockade therapy has been well-established, recent studies also suggest the involvement of non-canonical PD-1 signaling in blockade therapy. This review discusses the role of non-canonical PD-1 signaling in distinct cell types and explores how the available knowledge can improve PD-1 blockade immunotherapy, particularly in identifying novel biomarkers and combination treatment strategies.
Keywords: biomarkers; immunotherapy; programmed cell death 1 receptor; tumor; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures




Similar articles
-
What Happens to the Immune Microenvironment After PD-1 Inhibitor Therapy?Front Immunol. 2021 Dec 23;12:773168. doi: 10.3389/fimmu.2021.773168. eCollection 2021. Front Immunol. 2021. PMID: 35003090 Free PMC article. Review.
-
The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies.Nat Immunol. 2020 Nov;21(11):1346-1358. doi: 10.1038/s41590-020-0769-3. Epub 2020 Aug 31. Nat Immunol. 2020. PMID: 32868929
-
TOX-expressing terminally exhausted tumor-infiltrating CD8+ T cells are reinvigorated by co-blockade of PD-1 and TIGIT in bladder cancer.Cancer Lett. 2021 Feb 28;499:137-147. doi: 10.1016/j.canlet.2020.11.035. Epub 2020 Nov 27. Cancer Lett. 2021. PMID: 33249194
-
CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer.J Immunother Cancer. 2021 Jan;9(1):e001136. doi: 10.1136/jitc-2020-001136. J Immunother Cancer. 2021. PMID: 33452206 Free PMC article.
-
Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination.Int J Cancer. 2020 Sep 15;147(6):1509-1518. doi: 10.1002/ijc.32889. Epub 2020 Feb 18. Int J Cancer. 2020. PMID: 31997345 Review.
Cited by
-
When killers become thieves: Trogocytosed PD-1 inhibits NK cells in cancer.Sci Adv. 2022 Apr 15;8(15):eabj3286. doi: 10.1126/sciadv.abj3286. Epub 2022 Apr 13. Sci Adv. 2022. PMID: 35417234 Free PMC article.
-
PD-1 receptor outside the main paradigm: tumour-intrinsic role and clinical implications for checkpoint blockade.Br J Cancer. 2023 Oct;129(9):1409-1416. doi: 10.1038/s41416-023-02363-2. Epub 2023 Jul 20. Br J Cancer. 2023. PMID: 37474722 Free PMC article. Review.
-
sPGGM: a sample-perturbed Gaussian graphical model for identifying pre-disease stages and signaling molecules of disease progression.Natl Sci Rev. 2025 May 14;12(8):nwaf189. doi: 10.1093/nsr/nwaf189. eCollection 2025 Aug. Natl Sci Rev. 2025. PMID: 40635685 Free PMC article.
-
Development and pharmacokinetic assessment of a fully canine anti-PD-1 monoclonal antibody for comparative translational research in dogs with spontaneous tumors.MAbs. 2023 Jan-Dec;15(1):2287250. doi: 10.1080/19420862.2023.2287250. Epub 2023 Dec 4. MAbs. 2023. PMID: 38047502 Free PMC article.
-
Immunomodulatory Properties of Immune Checkpoint Inhibitors-More than Boosting T-Cell Responses?Cancers (Basel). 2022 Mar 28;14(7):1710. doi: 10.3390/cancers14071710. Cancers (Basel). 2022. PMID: 35406483 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
