Prospective development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors
- PMID: 33593828
- PMCID: PMC7888377
- DOI: 10.1136/jitc-2020-001845
Prospective development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors
Abstract
Background: The predictive power of novel biological markers for treatment response to immune checkpoint inhibitors (ICI) is still not satisfactory for the majority of patients with cancer. One should identify valid predictive markers in the peripheral blood, as this is easily available before and during treatment. The current interim analysis of patients of the ST-ICI cohort therefore focuses on the development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with metastatic cancer to ICI targeting the programmed cell death protein 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) axis.
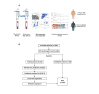
Methods: A total of 104 patients were prospectively enrolled. 54 immune cell subsets were prospectively analyzed in patients' peripheral blood by multicolor flow cytometry before treatment with ICI (pre-ICI; n=89), and after the first application of ICI (n=65). Pre-ICI, patients were randomly allocated to a training (n=56) and a validation cohort (n=33). Univariate Cox proportional hazards regression analysis and least absolute shrinkage and selection operator Cox model were used to create a predictive immune signature, which was also checked after the first ICI, to consider the dynamics of changes in the immune status.
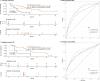
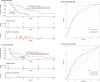
Results: Whole blood samples were provided by 89 patients pre-ICI and by 65 patients after the first ICI. We identified a LIPS which is based on five immune cell subtypes: CD14high monocytes, CD8+/PD-1+ T cells, plasmacytoid dendritic cells, neutrophils, and CD3+/CD56+/CD16+ natural killer (NK)T cells. The signature achieved a high accuracy (C-index 0.74 vs 0.71) for predicting overall survival (OS) benefit in both the training and the validation cohort. In both cohorts, the low-risk group had significantly longer OS than the high-risk group (HR 0.26, 95% CI 0.12 to 0.56, p=0.00025; HR 0.30, 95% CI 0.10 to 0.91, p=0.024, respectively). Regarding the whole cohort, LIPS also predicted progression-free survival (PFS). The identified LIPS was not affected by clinicopathological features with the exception of brain metastases. NKT cells and neutrophils of the LIPS can be used as dynamic predictive biomarkers for OS and PFS after first administration of the ICI.
Conclusion: Our study identified a predictive LIPS for survival of patients with cancer treated with PD-1/PD-L1 ICI, which is based on immune cell subsets in the peripheral whole blood.
Trial registration number: NCT03453892.
Keywords: biomarkers; immunotherapy; programmed cell death 1 receptor; tumor; tumor biomarkers; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MH reports conflict of interest with Merck Serono (advisory boards, honoraria for lectures, travel grants, research funding); MSD (advisory boards, travel grants, research funding); AstraZeneca (research funding); Novartis (research funding); BMS (advisory boards, honoraria for lectures); Teva (travel grants). USG received support for presentation activities for Dr Sennewald Medizintechnik GmbH, has received support for investigator initiated clinical studies (IITs) from MSD and AstraZeneca and contributed at Advisory Boards Meetings of AstraZeneca and Bristol-Myers Squibb. JGZ received support from AstraZeneca (travel grants). HM reports conflict of interest with Merck Serono (advisory boards, honoraria for lectures, travel grants); MSD (advisory boards, honoraria for lectures, travel grants); AstraZeneca (advisory boards, honoraria for lectures, travel grants); BMS (advisory boards, honoraria for lectures, travel grants). RS received travel and accommodation expenses from AstraZeneca. ED reports grants and honoraria from ROCHE GENENTECH, grants from SERVIER, grants and honoraria from ASTRAZENECA, MERCK SERONO, BMS and MSD, outside the submitted work. SR conflict of interest with AstraZeneca (research funding); MSD (research funding). ME conflict of interest with Diaceutics (employment, honoraria, advisory role, speakers’ bureau, travel expenses); AstraZeneca (honoraria, advisory role, speakers’ bureau, travel expenses); Roche (honoraria, travel expenses); MSD (honoraria, speakers’ bureau); GenomicHealth (honoraria, advisory role, speakers bureau, travel expenses); Astellas (honoraria, speakers’ bureau); Janssen-Cilag (honoraria, advisory role, research funding, travel expenses); Stratifyer (research funding, patents). RF conflict of interest with MSD (honoraria, advisory role, research funding, travel expenses); Fresenius (honoraria); BrainLab (honoraria); AstraZeneca (honoraria, advisory role, research funding, travel expenses); Merck Serono (advisory role, research funding, travel expenses); Novocure (advisory role, speakers’ bureau, research funding); Sennewald (speakers’ bureau, travel expenses).
Figures


References
-
- Burtness B, Harrington KJ, Greil R, et al. . KEYNOTE-048: phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (r/m HNSCC). Annals of Oncology 2018;29:viii729 10.1093/annonc/mdy424.045 - DOI
-
- Mok TSK, Wu Y-L, Kudaba I, et al. . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819–30. 10.1016/S0140-6736(18)32409-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
