Hospital outbreak of fluconazole-resistant Candida parapsilosis: arguments for clonal transmission and long-term persistence
- PMID: 33593841
- PMCID: PMC8092880
- DOI: 10.1128/AAC.02036-20
Hospital outbreak of fluconazole-resistant Candida parapsilosis: arguments for clonal transmission and long-term persistence
Abstract
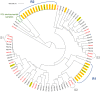
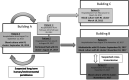
The worldwide emergence of multidrug-resistant pathogenic fungi is a threat to human health. At this very moment, an emergence of Candida parapsilosis isolates harbouring a resistance to fluconazole, one of the most popular antifungal drugs, is being described in several countries. We seek to better understanding the epidemiology, pathogenicity and transmission of resistant Candida parapsilosis Faced with an outbreak of invasive infections due to resistant isolates of C. parapsilosis, we performed a 7-year retrospective and prospective analysis of 283 C. parapsilosis isolates collected in 240 patients, among who 111 had invasive candidiasis. Study included review of hospital records, genotyping analysis and susceptibility testing that allow determining the type and outcome of infections, as well as the spatial and temporal spread of clusters. Overall the incidence of azole resistance was 7.5%. Genotyping analysis unveiled several previously undetected outbreaks and clonal spread of susceptible and resistant isolates over a long period of time. In comparison with susceptible isolates, resistant ones have a more restricted genetic diversity and seem to be more likely to spread and more frequently associated with invasive infections. In intensive care units, patients with invasive infections due to resistant isolates had poorer outcome (overall mortality at day 30 of 40%; 4/10) than susceptible ones (overall mortality at day 30 of 26.5%; 9/34). Our results suggest that the propensity of C. parapsilosis to spread on an epidemic fashion is underestimated, which warrants reinforced control and epidemiological survey of this species.
Copyright © 2021 American Society for Microbiology.
Figures
References
-
- Prigitano A, Cavanna C, Passera M, Gelmi M, Sala E, Ossi C, Grancini A, Calabro M, Bramati S, Tejada M, Lallitto F, Farina C, Rognoni V, Fasano MA, Pini B, Romano L, Cogliati M, Esposto MC, Tortorano AM. 2019. Evolution of fungemia in an Italian region. J Mycol Med 30:100906. 10.1016/j.mycmed.2019.100906. - DOI - PubMed
-
- Puig-Asensio M, Padilla B, Garnacho-Montero J, Zaragoza O, Aguado JM, Zaragoza R, Montejo M, Munoz P, Ruiz-Camps I, Cuenca-Estrella M, Almirante B, CANDIPOP Project, GEIH-GEMICOMED (SEIMC), REIPI. 2014. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect 20:O245–O254. 10.1111/1469-0691.12380. - DOI - PubMed
-
- Santolaya ME, Thompson L, Benadof D, Tapia C, Legarraga P, Cortes C, Rabello M, Valenzuela R, Rojas P, Rabagliati R, Chilean Invasive Mycosis Network. 2019. A prospective, multi-center study of Candida bloodstream infections in Chile. PLoS One 14:e0212924. 10.1371/journal.pone.0212924. - DOI - PMC - PubMed
-
- Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ng KP, Colombo A, Finquelievich J, Barnes R, Wadula J, Global Antifungal Surveillance Group. 2008. Geographic and temporal trends in isolation and antifungal susceptibility of Candida parapsilosis: a global assessment from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol 46:842–849. 10.1128/JCM.02122-07. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

