Antibiotic susceptibility of clinical Burkholderia pseudomallei isolates in northeast Thailand during 2015-2018 and the genomic characterization of β-lactam-resistant isolates
- PMID: 33593842
- PMCID: PMC8092913
- DOI: 10.1128/AAC.02230-20
Antibiotic susceptibility of clinical Burkholderia pseudomallei isolates in northeast Thailand during 2015-2018 and the genomic characterization of β-lactam-resistant isolates
Abstract
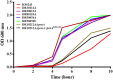
Melioidosis is an often fatal infection in tropical regions caused by an environmental bacterium, Burkholderia pseudomallei Current recommended melioidosis treatment requires intravenous β-lactam antibiotics such as ceftazidime (CAZ), meropenem (MEM) or amoxicillin-clavulanic acid (AMC) and oral trimethoprim-sulfamethoxazole. Emerging antibiotic resistance could lead to therapy failure and high mortality. We performed a prospective multicentre study in northeast Thailand during 2015-2018 to evaluate antibiotic susceptibility and characterize β-lactam resistance in clinical B. pseudomallei isolates. Collection of 1,317 B. pseudomallei isolates from patients with primary and relapse infections were evaluated for susceptibility to CAZ, imipenem (IPM), MEM and AMC. β-lactam resistant isolates were confirmed by broth microdilution method and characterized by whole genome sequence analysis, penA expression and β-lactamase activity. The resistant phenotype was verified via penA mutagenesis. All primary isolates were IPM-susceptible but we observed two CAZ-resistant and one CAZ-intermediate resistant isolates, two MEM-less susceptible isolates, one AMC-resistant and two AMC-intermediate resistant isolates. One of 13 relapse isolates was resistant to both CAZ and AMC. Two isolates were MEM-less susceptible. Strains DR10212A (primary) and DR50054E (relapse) were multi-drug resistant. Genomic and mutagenesis analyses supplemented with gene expression and β-lactamase analyses demonstrated that CAZ-resistant phenotype was caused by PenA variants: P167S (N=2) and penA amplification (N=1). Despite the high mortality rate in melioidosis, our study revealed that B. pseudomallei isolates had a low frequency of β-lactam resistance caused by penA alterations. Clinical data suggest that resistant variants may emerge in patients during antibiotic therapy and be associated with poor response to treatment.
Copyright © 2021 Fen et al.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

