In Vitro and In Vivo Characterization of Tebipenem (TBP), an Orally Active Carbapenem, against Biothreat Pathogens
- PMID: 33593844
- PMCID: PMC8092902
- DOI: 10.1128/AAC.02385-20
In Vitro and In Vivo Characterization of Tebipenem (TBP), an Orally Active Carbapenem, against Biothreat Pathogens
Abstract
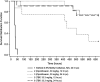
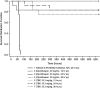
Bacillus anthracis and Yersinia pestis, causative pathogens for anthrax and plague, respectively, along with Burkholderia mallei and B. pseudomallei are potential bioterrorism threats. Tebipenem pivoxil hydrobromide (TBP HBr, formerly SPR994), is an orally available prodrug of tebipenem, a carbapenem with activity versus multidrug-resistant (MDR) gram-negative pathogens, including quinolone-resistant and extended-spectrum-β-lactamase-producing Enterobacterales. We evaluated the in vitro activity and in vivo efficacy of tebipenem against biothreat pathogens. Tebipenem was active in vitro against 30-strain diversity sets of B. anthracis, Y. pestis, B. mallei, and B. pseudomallei with minimum inhibitory concentration (MIC) values of 0.001 - 0.008 μg/ml for B. anthracis, ≤0.0005 - 0.03 μg/ml for Y. pestis, 0.25 - 1 μg/ml for B. mallei, and 1 - 4 μg/ml for B. pseudomallei In a B. anthracis murine model, all control animals died within 52 h post challenge. The survival rates in the groups treated with tebipenem were 75% and 73% when dosed at 12 h and 24 h post challenge, respectively. The survival rates in the positive control groups treated with ciprofloxacin were 75% and when dosed 12 h and 25% when dosed 24 h post challenge, respectively. Survival rates were significantly (p=0.0009) greater in tebipenem groups treated at 12 h and 24 h post challenge and in the ciprofloxacin group 12 h post-challenge vs. the vehicle-control group. For Y. pestis, survival rates for all animals in the tebipenem and ciprofloxacin groups were significantly (p<0.0001) greater than the vehicle-control group. These results support further development of tebipenem for treating biothreat pathogens.
Copyright © 2021 American Society for Microbiology.
Figures


Similar articles
-
The Class A β-Lactamase Produced by Burkholderia Species Compromises the Potency of Tebipenem against a Panel of Isolates from the United States.Antibiotics (Basel). 2022 May 17;11(5):674. doi: 10.3390/antibiotics11050674. Antibiotics (Basel). 2022. PMID: 35625319 Free PMC article.
-
In Vitro and In Vivo Activity of Omadacycline against Two Biothreat Pathogens, Bacillus anthracis and Yersinia pestis.Antimicrob Agents Chemother. 2017 Apr 24;61(5):e02434-16. doi: 10.1128/AAC.02434-16. Print 2017 May. Antimicrob Agents Chemother. 2017. PMID: 28223382 Free PMC article.
-
In Vitro and In Vivo Characterization of Tebipenem, an Oral Carbapenem.Antimicrob Agents Chemother. 2020 Jul 22;64(8):e02240-19. doi: 10.1128/AAC.02240-19. Print 2020 Jul 22. Antimicrob Agents Chemother. 2020. PMID: 32423950 Free PMC article.
-
Tebipenem pivoxil hydrobromide-No PICC, no problem!Pharmacotherapy. 2021 Sep;41(9):748-761. doi: 10.1002/phar.2614. Epub 2021 Aug 17. Pharmacotherapy. 2021. PMID: 34370326 Review.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
Cited by
-
Ceftibuten-Ledaborbactam Activity against Multidrug-Resistant and Extended-Spectrum-β-Lactamase-Positive Clinical Isolates of Enterobacterales from a 2018-2020 Global Surveillance Collection.Antimicrob Agents Chemother. 2022 Nov 15;66(11):e0093422. doi: 10.1128/aac.00934-22. Epub 2022 Oct 26. Antimicrob Agents Chemother. 2022. PMID: 36286518 Free PMC article.
-
The Class A β-Lactamase Produced by Burkholderia Species Compromises the Potency of Tebipenem against a Panel of Isolates from the United States.Antibiotics (Basel). 2022 May 17;11(5):674. doi: 10.3390/antibiotics11050674. Antibiotics (Basel). 2022. PMID: 35625319 Free PMC article.
-
Efficacy of ANTHRASIL (Anthrax Immune Globulin Intravenous (Human)) in rabbit and nonhuman primate models of inhalational anthrax: Data supporting approval under animal rule.PLoS One. 2023 Mar 17;18(3):e0283164. doi: 10.1371/journal.pone.0283164. eCollection 2023. PLoS One. 2023. PMID: 36930692 Free PMC article. Clinical Trial.
-
Plasma and Intrapulmonary Concentrations of Tebipenem following Oral Administration of Tebipenem Pivoxil Hydrobromide to Healthy Adult Subjects.Antimicrob Agents Chemother. 2022 Jul 19;66(7):e0059022. doi: 10.1128/aac.00590-22. Epub 2022 Jun 28. Antimicrob Agents Chemother. 2022. PMID: 35762796 Free PMC article. Clinical Trial.
References
-
- Rai B, Kaur J. 2013. Evidence-based forensic dentistry, p 149–152. Springer-Verlag, Berlin, Germany.
-
- Weiss S, Yitzhaki S, Shapira SC. 2015. Lessons to be learned from recent biosafety incidents in the United States. Isr Med Assoc J 17:269–273. - PubMed
-
- Centers for Disease Control and Prevention. 2018. Bioterrorism agents/diseases. Centers for Disease Control and Prevention, Atlanta, GA. https://emergency.cdc.gov/agent/agentlist-category.asp.
LinkOut - more resources
Full Text Sources
Other Literature Sources

