Rod Photoreceptors Avoid Saturation in Bright Light by the Movement of the G Protein Transducin
- PMID: 33593858
- PMCID: PMC8051685
- DOI: 10.1523/JNEUROSCI.2817-20.2021
Rod Photoreceptors Avoid Saturation in Bright Light by the Movement of the G Protein Transducin
Abstract
Rod photoreceptors can be saturated by exposure to bright background light, so that no flash superimposed on the background can elicit a detectable response. This phenomenon, called increment saturation, was first demonstrated psychophysically by Aguilar and Stiles and has since been shown in many studies to occur in single rods. Recent experiments indicate, however, that rods may be able to avoid saturation under some conditions of illumination. We now show in ex vivo electroretinogram and single-cell recordings that in continuous and prolonged exposure even to very bright light, the rods of mice from both sexes recover as much as 15% of their dark current and that responses can persist for hours. In parallel to recovery of outer segment current is an ∼10-fold increase in the sensitivity of rod photoresponses. This recovery is decreased in transgenic mice with reduced light-dependent translocation of the G protein transducin. The reduction in outer-segment transducin together with a novel mechanism of visual-pigment regeneration within the rod itself enable rods to remain responsive over the whole of the physiological range of vision. In this way, rods are able to avoid an extended period of transduction channel closure, which is known to cause photoreceptor degeneration.SIGNIFICANCE STATEMENT Rods are initially saturated in bright light so that no flash superimposed on the background can elicit a detectable response. Frederiksen and colleagues show in whole retina and single-cell recordings that, if the background light is prolonged, rods slowly recover and can continue to produce significant responses over the entire physiological range of vision. Response recovery occurs by translocation of the G protein transducin from the rod outer to the inner segment, together with a novel mechanism of visual-pigment regeneration within the rod itself. Avoidance of saturation in bright light may be one of the principal mechanisms the retina uses to keep rod outer-segment channels from ever closing for too long a time, which is known to produce photoreceptor degeneration.
Keywords: G protein; adaptation; retina; rod photoreceptor; saturation; visual pigment.
Copyright © 2021 the authors.
Figures



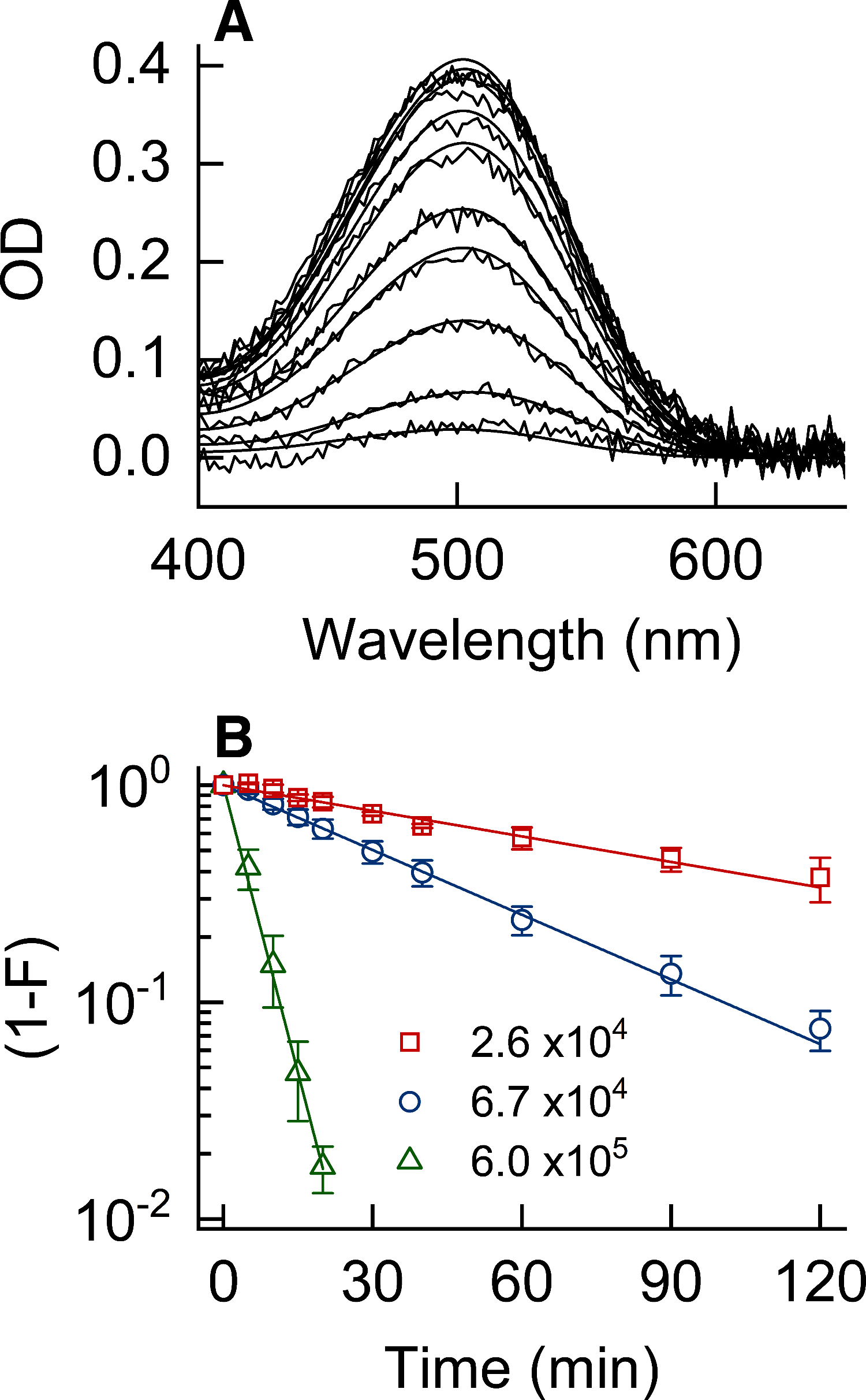

References
-
- Aguilar M, Stiles WS (1954) Saturation of the rod mechanism of the retina at high levels of stimulation. Optica Acta 1:59–65. 10.1080/713818657 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
