Network Asynchrony Underlying Increased Broadband Gamma Power
- PMID: 33593859
- PMCID: PMC8018896
- DOI: 10.1523/JNEUROSCI.2250-20.2021
Network Asynchrony Underlying Increased Broadband Gamma Power
Abstract
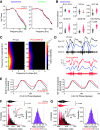
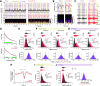
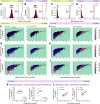
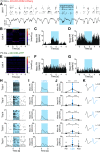
Synchronous activity of cortical inhibitory interneurons expressing parvalbumin (PV) underlies expression of cortical γ rhythms. Paradoxically, deficient PV inhibition is associated with increased broadband γ power in the local field potential. Increased baseline broadband γ is also a prominent characteristic in schizophrenia and a hallmark of network alterations induced by NMDAR antagonists, such as ketamine. Whether enhanced broadband γ is a true rhythm, and if so, whether rhythmic PV inhibition is involved or not, is debated. Asynchronous and increased firing activities are thought to contribute to broadband power increases spanning the γ band. Using male and female mice lacking NMDAR activity specifically in PV neurons to model deficient PV inhibition, we here show that neuronal activity with decreased synchronicity is associated with increased prefrontal broadband γ power. Specifically, reduced spike time precision and spectral leakage of spiking activity because of higher firing rates (spike "contamination") affect the broadband γ band. Desynchronization was evident at multiple time scales, with reduced spike entrainment to the local field potential, reduced cross-frequency coupling, and fragmentation of brain states. Local application of S(+)-ketamine in (control) mice with intact NMDAR activity in PV neurons triggered network desynchronization and enhanced broadband γ power. However, our investigations suggest that disparate mechanisms underlie increased broadband γ power caused by genetic alteration of PV interneurons and ketamine-induced power increases in broadband γ. Our study confirms that enhanced broadband γ power can arise from asynchronous activities and demonstrates that long-term deficiency of PV inhibition can be a contributor.SIGNIFICANCE STATEMENT Brain oscillations are fundamental to the coordination of neuronal activity across neurons and structures. γ oscillations (30-80 Hz) have received particular attention through their association with perceptual and cognitive processes. Synchronous activity of inhibitory parvalbumin (PV) interneurons generates cortical γ oscillation, but, paradoxically, PV neuron deficiency is associated with increases in γ oscillations. We here reconcile this conundrum and show how deficient PV inhibition can lead to increased and asynchronous excitatory firing, contaminating the local field potential and manifesting as increased γ power. Thus, increased γ power does not always reflect a genuine rhythm. Further, we show that ketamine-induced γ increases are caused by separate network mechanisms.
Keywords: DOWN and UP states; NMDAR; PFC; asynchrony; broadband gamma; parvalbumin.
Copyright © 2021 the authors.
Figures


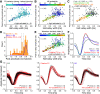
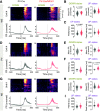

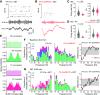
Similar articles
-
Adult trkB Signaling in Parvalbumin Interneurons is Essential to Prefrontal Network Dynamics.J Neurosci. 2021 Apr 7;41(14):3120-3141. doi: 10.1523/JNEUROSCI.1848-20.2021. Epub 2021 Feb 16. J Neurosci. 2021. PMID: 33593856 Free PMC article.
-
Global Grin2a loss causes divergent impairments to PV+ and SST+ interneurons and alters gamma oscillations in prefrontal cortex.Neurobiol Dis. 2025 Aug;212:106977. doi: 10.1016/j.nbd.2025.106977. Epub 2025 May 26. Neurobiol Dis. 2025. PMID: 40436282
-
NMDA receptors on parvalbumin-positive interneurons and pyramidal neurons both contribute to MK-801 induced gamma oscillatory disturbances: Complex relationships with behaviour.Neurobiol Dis. 2020 Feb;134:104625. doi: 10.1016/j.nbd.2019.104625. Epub 2019 Nov 28. Neurobiol Dis. 2020. PMID: 31786371
-
Parvalbumin interneuron deficits in schizophrenia.Eur Neuropsychopharmacol. 2024 May;82:44-52. doi: 10.1016/j.euroneuro.2024.02.010. Epub 2024 Mar 14. Eur Neuropsychopharmacol. 2024. PMID: 38490084 Free PMC article. Review.
-
Abnormal Gamma Oscillations in N-Methyl-D-Aspartate Receptor Hypofunction Models of Schizophrenia.Biol Psychiatry. 2016 May 1;79(9):716-726. doi: 10.1016/j.biopsych.2015.07.005. Epub 2015 Jul 17. Biol Psychiatry. 2016. PMID: 26281716 Free PMC article. Review.
Cited by
-
Trends in big data analyses by multicenter collaborative translational research in psychiatry.Psychiatry Clin Neurosci. 2022 Jan;76(1):1-14. doi: 10.1111/pcn.13311. Psychiatry Clin Neurosci. 2022. PMID: 34716732 Free PMC article. Review.
-
Recent Advances and Future Directions in Brain MR Imaging Studies in Schizophrenia: Toward Elucidating Brain Pathology and Developing Clinical Tools.Magn Reson Med Sci. 2022 Oct 1;21(4):539-552. doi: 10.2463/mrms.rev.2021-0050. Epub 2021 Aug 19. Magn Reson Med Sci. 2022. PMID: 34408115 Free PMC article.
-
Decoupling of cortical activity from behavioral state following administration of the classic psychedelic DOI.Neuropharmacology. 2024 Oct 1;257:110030. doi: 10.1016/j.neuropharm.2024.110030. Epub 2024 Jun 6. Neuropharmacology. 2024. PMID: 38851531
-
Response outcome gates the effect of spontaneous cortical state fluctuations on perceptual decisions.Elife. 2023 May 17;12:e81774. doi: 10.7554/eLife.81774. Elife. 2023. PMID: 37195029 Free PMC article.
-
Gamma Oscillations as a Biomarker of Neural Circuit Function in Psychosis: Where Are We, and Where Do We Go from Here?Adv Neurobiol. 2024;40:321-349. doi: 10.1007/978-3-031-69491-2_12. Adv Neurobiol. 2024. PMID: 39562450 Review.
References
-
- Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, Morita S, Featherstone RE, Ortinski PI, Gandal MJ, Lin R, Liang Y, Gur RE, Carlson GC, Hahn CG, Siegel SJ (2014) Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology 39:1603–1613. 10.1038/npp.2014.7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
