Quantifying Treatment-Related Fluctuations in CIDP: Results of the GRIPPER Study
- PMID: 33593867
- PMCID: PMC8105962
- DOI: 10.1212/WNL.0000000000011703
Quantifying Treatment-Related Fluctuations in CIDP: Results of the GRIPPER Study
Abstract
Objective: The objective of this study was to explore the extent of IV immunoglobulin (IVIG) treatment-related fluctuations (TRFs) by using home collection of daily grip strength in patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) and to use that information to develop evidence-based treatment optimization strategies.
Methods: This prospective observational study included 25 patients with well-defined CIDP. Participants recorded grip strength daily for 6 months. Disability and gait metrics were collected weekly. Serum immunoglobulin G levels were obtained at peak, trough, and midcycle IVIG intervals. Day-to-day grip strength changes <10% were considered random. To identify patients with TRFs, 3-day averaged grip strength was calculated on each consecutive day after an IVIG infusion. TRFs were defined as ≥10% 3-day averaged grip strength difference compared to the pre-IVIG baseline.
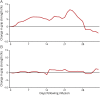
Results: Participants successfully recorded grip strength on all but 9% of recordable days. Twelve patients (48%) were classified as low/no fluctuaters and 13 (52%) as frequent fluctuaters. In the frequent fluctuating group, grip strength improved over 1 week and thereafter was relatively stable until the third week after infusion. Grip strength was significantly correlated with measures of disability.
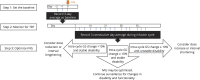
Conclusions: Grip strength collection by patients at home is reliable, valid, and feasible. A change in grip strength by ≥10% is a useful, practical, and evidence-based approach that may be used to identify clinically meaningful TRFs. From these data, we propose a treatment optimization strategy for patients with CIDP on chronic IVIG that may be applied to routine clinic care during both face-to-face and virtual video or telephone patient encounters.
Trial registration information: ClinicalTrials.gov Identifier: NCT02414490.
© 2021 American Academy of Neurology.
Figures


References
-
- Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society–first revision. Eur J Neurol 2010;17:356–363. - PubMed
-
- Rajabally YA, Simpson BS, Beri S, Bankart J, Gosalakkal JA. Epidemiologic variability of chronic inflammatory demyelinating polyneuropathy with different diagnostic criteria: study of a UK population. Muscle Nerve 2009;39:432–438. - PubMed
-
- Gorson KC, van Schaik IN, Merkies IS, et al. Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst 2010;15:326–333. - PubMed
-
- Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 2008;7:136–144. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
