High Detection Rates of Pancreatic Cancer Across Stages by Plasma Assay of Novel Methylated DNA Markers and CA19-9
- PMID: 33593879
- PMCID: PMC8102343
- DOI: 10.1158/1078-0432.CCR-20-0235
High Detection Rates of Pancreatic Cancer Across Stages by Plasma Assay of Novel Methylated DNA Markers and CA19-9
Abstract
Purpose: We have previously identified tissue methylated DNA markers (MDMs) associated with pancreatic ductal adenocarcinoma (PDAC). In this case-control study, we aimed to assess the diagnostic performance of plasma MDMs for PDAC.
Experimental design: Thirteen MDMs (GRIN2D, CD1D, ZNF781, FER1L4, RYR2, CLEC11A, AK055957, LRRC4, GH05J042948, HOXA1, PRKCB, SHISA9, and NTRK3) were identified on the basis of selection criteria applied to results of prior tissue experiments and assays were optimized in plasma. Next, 340 plasma samples (170 PDAC cases and 170 controls) were assayed using target enrichment long-probe quantitative amplified signal method. Initially, 120 advanced-stage PDAC cases and 120 healthy controls were used to train a prediction algorithm at 97.5% specificity using random forest modeling. Subsequently, the locked algorithm derived from the training set was applied to an independent blinded test set of 50 early-stage PDAC cases and 50 controls. Finally, data from all 340 patients were combined, and cross-validated.
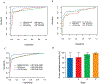
Results: The cross-validated area under the receiver operating characteristic curve (AUC) for the training set was 0.93 (0.89-0.96) for the MDM panel alone, 0.91 (95% confidence interval, 0.87-0.96) for carbohydrate antigen 19-9 (CA19-9) alone, and 0.99 (0.98-1) for the combined MDM-CA19-9 panel. In the test set of early-stage PDAC, the AUC for MDMs alone was 0.84 (0.76-0.92), CA19-9 alone was 0.87 (0.79-0.94), and combined MDM-CA19-9 panel was 0.90 (0.84-0.97) significantly better compared with either MDMs alone or CA19-9 alone (P = 0.0382 and 0.0490, respectively). At a preset specificity of 97.5%, the sensitivity for the combined panel in the test set was 80% (28%-99%) for stage I disease and 82% (68%-92%) for stage II disease. Using the combined datasets, the cross-validated AUC was 0.9 (0.86-0.94) for the MDM panel alone and 0.89 for CA19-9 alone (0.84-0.93) versus 0.97 (0.94-0.99) for the combined MDM-CA19-9 panel (P ≤ 0.0001). Overall, cross-validated sensitivity of MDM-CA19-9 panel was 92% (83%-98%), with an observed specificity of 92% at the preset specificity of 97.5%.
Conclusions: Plasma MDMs in combination with CA19-9 detect PDAC with significantly higher accuracy compared with either biomarker individually.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures

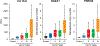
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68:7–30. - PubMed
-
- Ishikawa O, Ohigashi H, Imaoka S, Nakaizumi A, Uehara H, Kitamura T, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter--collective review of Japanese case reports. Hepato-gastroenterology. 1999;46:8–15. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69:7–34. - PubMed
-
- Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. Jama. 2019;322:438–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

