Modulation of α1β3γ2 GABAA receptors expressed in X. laevis oocytes using a propofol photoswitch tethered to the transmembrane helix
- PMID: 33593898
- PMCID: PMC7923644
- DOI: 10.1073/pnas.2008178118
Modulation of α1β3γ2 GABAA receptors expressed in X. laevis oocytes using a propofol photoswitch tethered to the transmembrane helix
Abstract
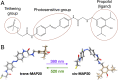
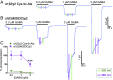
Tethered photoswitches are molecules with two photo-dependent isomeric forms, each with different actions on their biological targets. They include reactive chemical groups capable of covalently binding to their target. Our aim was to develop a β-subunit-tethered propofol photoswitch (MAP20), as a tool to better study the mechanism of anesthesia through the GABAA α1β3γ2 receptor. We used short spacers between the tether (methanethiosulfonate), the photosensitive moiety (azobenzene), and the ligand (propofol), to allow a precise tethering adjacent to the putative propofol binding site at the β+α- interface of the receptor transmembrane helices (TMs). First, we used molecular modeling to identify possible tethering sites in β3TM3 and α1TM1, and then introduced cysteines in the candidate positions. Two mutant subunits [β3(M283C) and α1(V227C)] showed photomodulation of GABA responses after incubation with MAP20 and illumination with lights at specific wavelengths. The α1β3(M283C)γ2 receptor showed the greatest photomodulation, which decreased as GABA concentration increased. The location of the mutations that produced photomodulation confirmed that the propofol binding site is located in the β+α- interface close to the extracellular side of the transmembrane helices. Tethering the photoswitch to cysteines introduced in the positions homologous to β3M283 in two other subunits (α1W288 and γ2L298) also produced photomodulation, which was not entirely reversible, probably reflecting the different nature of each interface. The results are in agreement with a binding site in the β+α- interface for the anesthetic propofol.
Keywords: anesthetic; azobenzene; methanethiosulfonate; molecular modeling; optopharmcology.
Conflict of interest statement
The authors declare no competing interest.
Figures


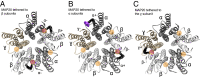
Similar articles
-
The role of the GABA(A) receptor alpha1 subunit N-terminal extracellular domain in propofol potentiation of chloride current.Neuropharmacology. 1997 Nov-Dec;36(11-12):1611-21. doi: 10.1016/s0028-3908(97)00180-9. Neuropharmacology. 1997. PMID: 9517432
-
The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis oocytes: a comparative study.Br J Pharmacol. 1997 Dec;122(8):1707-19. doi: 10.1038/sj.bjp.0701563. Br J Pharmacol. 1997. PMID: 9422818 Free PMC article.
-
Reduced effect of propofol at human {alpha}1{beta}2(N289M){gamma}2 and {alpha}2{beta}3(N290M){gamma}2 mutant GABA(A) receptors.Br J Anaesth. 2010 Apr;104(4):472-81. doi: 10.1093/bja/aeq023. Epub 2010 Feb 23. Br J Anaesth. 2010. PMID: 20179014
-
Defining the propofol binding site location on the GABAA receptor.Mol Pharmacol. 2004 Jan;65(1):68-76. doi: 10.1124/mol.65.1.68. Mol Pharmacol. 2004. PMID: 14722238
-
Multiple Non-Equivalent Interfaces Mediate Direct Activation of GABAA Receptors by Propofol.Curr Neuropharmacol. 2016;14(7):772-80. doi: 10.2174/1570159x14666160202121319. Curr Neuropharmacol. 2016. PMID: 26830963 Free PMC article. Review.
Cited by
-
Photopharmacology of Ion Channels through the Light of the Computational Microscope.Int J Mol Sci. 2021 Nov 8;22(21):12072. doi: 10.3390/ijms222112072. Int J Mol Sci. 2021. PMID: 34769504 Free PMC article. Review.
-
Massive Activation of GABAA Receptors: Rundown, Ionic and Neurodegenerative Consequences.Biomolecules. 2025 Jul 13;15(7):1003. doi: 10.3390/biom15071003. Biomolecules. 2025. PMID: 40723875 Free PMC article. Review.
-
Remimazolam Tosylate Combined with Low-Dose Propofol Improves Sedation and Safety in Hysteroscopy.Drug Des Devel Ther. 2022 Nov 29;16:4101-4108. doi: 10.2147/DDDT.S390403. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36471692 Free PMC article. Clinical Trial.
-
Light-Activated Agonist-Potentiator of GABAA Receptors for Reversible Neuroinhibition in Wildtype Mice.J Am Chem Soc. 2024 Oct 23;146(42):28822-28831. doi: 10.1021/jacs.4c08446. Epub 2024 Oct 9. J Am Chem Soc. 2024. PMID: 39383450 Free PMC article.
-
Phenols and GABAA receptors: from structure and molecular mechanisms action to neuropsychiatric sequelae.Front Pharmacol. 2024 Jan 18;15:1272534. doi: 10.3389/fphar.2024.1272534. eCollection 2024. Front Pharmacol. 2024. PMID: 38303988 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

