Competitive binding of independent extension and retraction motors explains the quantitative dynamics of type IV pili
- PMID: 33593905
- PMCID: PMC7923367
- DOI: 10.1073/pnas.2014926118
Competitive binding of independent extension and retraction motors explains the quantitative dynamics of type IV pili
Abstract
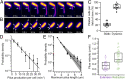
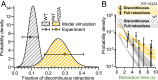
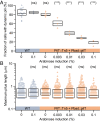
Type IV pili (TFP) function through cycles of extension and retraction. The coordination of these cycles remains mysterious due to a lack of quantitative measurements of multiple features of TFP dynamics. Here, we fluorescently label TFP in the pathogen Pseudomonas aeruginosa and track full extension and retraction cycles of individual filaments. Polymerization and depolymerization dynamics are stochastic; TFP are made at random times and extend, pause, and retract for random lengths of time. TFP can also pause for extended periods between two extension or two retraction events in both wild-type cells and a slowly retracting PilT mutant. We developed a biophysical model based on the stochastic binding of two dedicated extension and retraction motors to the same pilus machine that predicts the observed features of the data with no free parameters. We show that only a model in which both motors stochastically bind and unbind to the pilus machine independent of the piliation state of the machine quantitatively explains the experimentally observed pilus production rate. In experimental support of this model, we show that the abundance of the retraction motor dictates the pilus production rate and that PilT is bound to pilus machines even in their unpiliated state. Together, the strong quantitative agreement of our model with a variety of experiments suggests that the entire repetitive cycle of pilus extension and retraction is coordinated by the competition of stochastic motor binding to the pilus machine, and that the retraction motor is the major throttle for pilus production.
Keywords: competitive binding; molecular motor; pilus dynamics; type IV pili.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Burrows L. L., Pseudomonas aeruginosa twitching motility: Type IV pili in action. Annu. Rev. Microbiol. 66, 493–520 (2012). - PubMed
-
- Craig L., Forest K. T., Maier B., Type IV pili: Dynamics, biophysics and functional consequences. Nat. Rev. Microbiol. 17, 429–440 (2019). - PubMed
-
- Merz A. J., So M., Sheetz M. P., Pilus retraction powers bacterial twitching motility. Nature 407, 98–102 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

