Nile Red fluorescence spectroscopy reports early physicochemical changes in myelin with high sensitivity
- PMID: 33593907
- PMCID: PMC7923366
- DOI: 10.1073/pnas.2016897118
Nile Red fluorescence spectroscopy reports early physicochemical changes in myelin with high sensitivity
Abstract
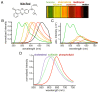
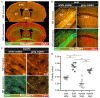
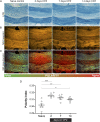
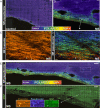
The molecular composition of myelin membranes determines their structure and function. Even minute changes to the biochemical balance can have profound consequences for axonal conduction and the synchronicity of neural networks. Hypothesizing that the earliest indication of myelin injury involves changes in the composition and/or polarity of its constituent lipids, we developed a sensitive spectroscopic technique for defining the chemical polarity of myelin lipids in fixed frozen tissue sections from rodent and human. The method uses a simple staining procedure involving the lipophilic dye Nile Red, whose fluorescence spectrum varies according to the chemical polarity of the microenvironment into which the dye embeds. Nile Red spectroscopy identified histologically intact yet biochemically altered myelin in prelesioned tissues, including mouse white matter following subdemyelinating cuprizone intoxication, as well as normal-appearing white matter in multiple sclerosis brain. Nile Red spectroscopy offers a relatively simple yet highly sensitive technique for detecting subtle myelin changes.
Keywords: cuprizone; fluorescence spectroscopy; lipids; multiple sclerosis; spectral confocal microscopy.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

