The ecdysone-induced protein 93 is a key factor regulating gonadotrophic cycles in the adult female mosquito Aedes aegypti
- PMID: 33593917
- PMCID: PMC7923369
- DOI: 10.1073/pnas.2021910118
The ecdysone-induced protein 93 is a key factor regulating gonadotrophic cycles in the adult female mosquito Aedes aegypti
Abstract
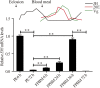
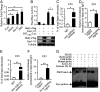
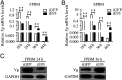
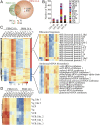
Repeated blood feedings are required for adult female mosquitoes to maintain their gonadotrophic cycles, enabling them to be important pathogen carriers of human diseases. Elucidating the molecular mechanism underlying developmental switches between these mosquito gonadotrophic cycles will provide valuable insight into mosquito reproduction and could aid in the identification of targets to disrupt these cycles, thereby reducing disease transmission. We report here that the transcription factor ecdysone-induced protein 93 (E93), previously implicated in insect metamorphic transitions, plays a key role in determining the gonadotrophic cyclicity in adult females of the major arboviral vector Aedes aegypti Expression of the E93 gene in mosquitoes is down-regulated by juvenile hormone (JH) and up-regulated by 20-hydroxyecdysone (20E). We find that E93 controls Hormone Receptor 3 (HR3), the transcription factor linked to the termination of reproductive cycles. Moreover, knockdown of E93 expression via RNAi impaired fat body autophagy, suggesting that E93 governs autophagy-induced termination of vitellogenesis. E93 RNAi silencing prior to the first gonadotrophic cycle affected normal progression of the second cycle. Finally, transcriptomic analysis showed a considerable E93-dependent decline in the expression of genes involved in translation and metabolism at the end of a reproductive cycle. In conclusion, our data demonstrate that E93 acts as a crucial factor in regulating reproductive cycle switches in adult female mosquitoes.
Keywords: autophagy; ecdysone-induced protein 93; juvenile hormone; mosquito; reproduction.
Conflict of interest statement
The authors declare no competing interest.
Figures



Comment in
-
Adult specifier E93 takes control of reproductive cyclicity in mosquitoes.Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2102059118. doi: 10.1073/pnas.2102059118. Proc Natl Acad Sci U S A. 2021. PMID: 33674423 Free PMC article. No abstract available.
Similar articles
-
A critical role of the nuclear receptor HR3 in regulation of gonadotrophic cycles of the mosquito Aedes aegypti.PLoS One. 2012;7(9):e45019. doi: 10.1371/journal.pone.0045019. Epub 2012 Sep 26. PLoS One. 2012. PMID: 23049766 Free PMC article.
-
Expression of the early-late gene encoding the nuclear receptor HR3 suggests its involvement in regulating the vitellogenic response to ecdysone in the adult mosquito.Mol Cell Endocrinol. 2000 Feb 25;160(1-2):25-37. doi: 10.1016/s0303-7207(99)00253-1. Mol Cell Endocrinol. 2000. PMID: 10715536
-
Programmed autophagy in the fat body of Aedes aegypti is required to maintain egg maturation cycles.PLoS One. 2011;6(11):e25502. doi: 10.1371/journal.pone.0025502. Epub 2011 Nov 17. PLoS One. 2011. PMID: 22125592 Free PMC article.
-
Krüppel homolog 1 and E93: The doorkeeper and the key to insect metamorphosis.Arch Insect Biochem Physiol. 2020 Mar;103(3):e21609. doi: 10.1002/arch.21609. Epub 2019 Aug 6. Arch Insect Biochem Physiol. 2020. PMID: 31385626 Review.
-
Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways.Front Physiol. 2014 Mar 20;5:103. doi: 10.3389/fphys.2014.00103. eCollection 2014. Front Physiol. 2014. PMID: 24688471 Free PMC article. Review.
Cited by
-
Polygenic adaptation from standing genetic variation allows rapid ecotype formation.Elife. 2023 Feb 28;12:e82824. doi: 10.7554/eLife.82824. Elife. 2023. PMID: 36852484 Free PMC article.
-
Ad libitum consumption of protein- or peptide-sucrose solutions stimulates egg formation by prolonging the vitellogenic phase of oogenesis in anautogenous mosquitoes.Parasit Vectors. 2022 Apr 12;15(1):127. doi: 10.1186/s13071-022-05252-4. Parasit Vectors. 2022. PMID: 35413939 Free PMC article.
-
The AaFoxA factor regulates female reproduction through chromatin remodeling in the mosquito vector Aedes aegypti.Proc Natl Acad Sci U S A. 2025 Mar 4;122(9):e2411758122. doi: 10.1073/pnas.2411758122. Epub 2025 Feb 24. Proc Natl Acad Sci U S A. 2025. PMID: 39993202 Free PMC article.
-
Research Progress on the Regulation of Autophagy and Apoptosis in Insects by Sterol Hormone 20-Hydroxyecdysone.Insects. 2023 Nov 12;14(11):871. doi: 10.3390/insects14110871. Insects. 2023. PMID: 37999070 Free PMC article. Review.
-
Aspirin Inhibition of Prostaglandin Synthesis Impairs Mosquito Egg Development.Cells. 2022 Dec 16;11(24):4092. doi: 10.3390/cells11244092. Cells. 2022. PMID: 36552860 Free PMC article.
References
-
- McNeil C. J., Shetty A. K., Zika virus: A serious global health threat. J. Trop. Pediatr. 63, 242–248 (2017). - PubMed
-
- Barrett A. D., Higgs S., Yellow fever: A disease that has yet to be conquered. Annu. Rev. Entomol. 52, 209–229 (2007). - PubMed
-
- Roy S., Saha T. T., Zou Z., Raikhel A. S., Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 63, 489–511 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

