Light Signaling Regulates Aspergillus niger Biofilm Formation by Affecting Melanin and Extracellular Polysaccharide Biosynthesis
- PMID: 33593965
- PMCID: PMC8545115
- DOI: 10.1128/mBio.03434-20
Light Signaling Regulates Aspergillus niger Biofilm Formation by Affecting Melanin and Extracellular Polysaccharide Biosynthesis
Abstract
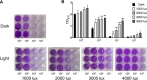
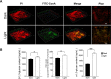
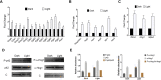
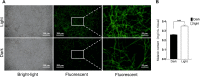
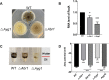
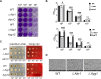
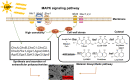
Light is an important signal source in nature, which regulates the physiological cycle, morphogenetic pathways, and secondary metabolites of fungi. As an external pressure on Aspergillus niger, light signaling transmits stress signals into the cell via the mitogen-activated protein kinase (MAPK) signaling pathway. Studying the effect of light on the biofilm of A. niger will provide a theoretical basis for light in the cultivation of filamentous fungi and industrial applications. Here, the characterization of A. niger biofilm under different light intensities confirmed the effects of light signaling. Our results indicated that A. niger intensely accumulated protective mycelial melanin under light illumination. We also discovered that the RlmA transcription factor in the MAPK signaling pathway is activated by light signaling to promote the synthesis of melanin, chitin, and other exopolysaccharides. However, the importance of melanin to A. niger biofilm is rarely reported; therefore, we knocked out key genes of the melanin biosynthetic pathway-Abr1 and Ayg1 Changes in hydrophobicity and electrostatic forces resulted in the decrease of biofilm caused by the decrease of melanin in mutants.IMPORTANCE As an important industrial filamentous fungus, Aspergillus niger can perceive light. The link between light signaling and A. niger biofilm is worthy of further study since reports are lacking in this area. This study found that light signaling promotes biofilm production in A. niger, wherein melanin plays an important role. It was further discovered that the RlmA transcription factor in the mitogen-activated protein kinase (MAPK) signaling pathway was mediated by light signaling to promote the synthesis of melanin and extracellular polysaccharides. These findings set the stage for light signal regulation of biofilm in filamentous fungi and provide a theoretical basis for the development of a new light-controlled biofilm method to improve biofilm-based industrial fermentation.
Keywords: Aspergillus niger; MAPK signaling pathway; biofilm; light signaling; melanin.
Copyright © 2021 Sun et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
