A Novel Mode of Photoprotection Mediated by a Cysteine Residue in the Chlorophyll Protein IsiA
- PMID: 33593975
- PMCID: PMC8545134
- DOI: 10.1128/mBio.03663-20
A Novel Mode of Photoprotection Mediated by a Cysteine Residue in the Chlorophyll Protein IsiA
Abstract
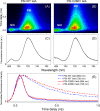
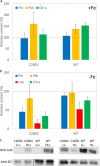
Oxygenic photosynthetic organisms have evolved a multitude of mechanisms for protection against high-light stress. IsiA, a chlorophyll a-binding cyanobacterial protein, serves as an accessory antenna complex for photosystem I. Intriguingly, IsiA can also function as an independent pigment protein complex in the thylakoid membrane and facilitate the dissipation of excess energy, providing photoprotection. The molecular basis of the IsiA-mediated excitation quenching mechanism remains poorly understood. In this study, we demonstrate that IsiA uses a novel cysteine-mediated process to quench excitation energy. The single cysteine in IsiA in the cyanobacterium Synechocystis sp. strain PCC 6803 was converted to a valine. Ultrafast fluorescence spectroscopic analysis showed that this single change abolishes the excitation energy quenching ability of IsiA, thus providing direct evidence of the crucial role of this cysteine residue in energy dissipation from excited chlorophylls. Under stress conditions, the mutant cells exhibited enhanced light sensitivity, indicating that the cysteine-mediated quenching process is critically important for photoprotection.IMPORTANCE Cyanobacteria, oxygenic photosynthetic microbes, constantly experience varying light regimes. Light intensities higher than those that saturate the photosynthetic capacity of the organism often lead to redox damage to the photosynthetic apparatus and often cell death. To meet this challenge, cyanobacteria have developed a number of strategies to modulate light absorption and dissipation to ensure maximal photosynthetic productivity and minimal photodamage to cells under extreme light conditions. In this communication, we have determined the critical role of a novel cysteine-mediated mechanism for light energy dissipation in the chlorophyll protein IsiA.
Keywords: Synechocystis; cyanobacteria; energy dissipation; photoprotection; photosynthesis.
Copyright © 2021 Chen et al.
Figures





References
-
- Martin JH, Fitzwater SE. 1988. Iron-deficiency limits phytoplankton growth in the Northeast Pacific Subarctic. Nature 331:341–343. doi: 10.1038/331341a0. - DOI
-
- Moore CM, Mills MM, Arrigo KR, Berman-Frank I, Bopp L, Boyd PW, Galbraith ED, Geider RJ, Guieu C, Jaccard SL, Jickells TD, La Roche J, Lenton TM, Mahowald NM, Marañón E, Marinov I, Moore JK, Nakatsuka T, Oschlies A, Saito MA, Thingstad TF, Tsuda A, Ulloa O. 2013. Processes and patterns of oceanic nutrient limitation. Nat Geosci 6:701–710. doi: 10.1038/ngeo1765. - DOI
-
- Vrede T, Tranvik LJ. 2006. Iron constraints on planktonic primary production in oligotrophic lakes. Ecosystems 9:1094–1105. doi: 10.1007/s10021-006-0167-1. - DOI
-
- North R, Guildford S, Smith R, Havens S, Twiss M. 2007. Evidence for phosphorus, nitrogen, and iron colimitation of phytoplankton communities in Lake Erie. Limnol Oceanogr 52:315–328. doi: 10.4319/lo.2007.52.1.0315. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
