Structural Characterization of Diazabicyclooctane β-Lactam "Enhancers" in Complex with Penicillin-Binding Proteins PBP2 and PBP3 of Pseudomonas aeruginosa
- PMID: 33593978
- PMCID: PMC8545096
- DOI: 10.1128/mBio.03058-20
Structural Characterization of Diazabicyclooctane β-Lactam "Enhancers" in Complex with Penicillin-Binding Proteins PBP2 and PBP3 of Pseudomonas aeruginosa
Abstract
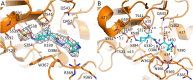
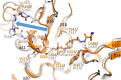
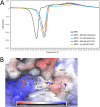
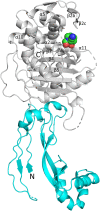
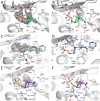
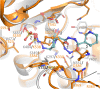
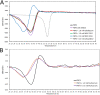
Multidrug-resistant (MDR) pathogens pose a significant public health threat. A major mechanism of resistance expressed by MDR pathogens is β-lactamase-mediated degradation of β-lactam antibiotics. The diazabicyclooctane (DBO) compounds zidebactam and WCK 5153, recognized as β-lactam "enhancers" due to inhibition of Pseudomonas aeruginosa penicillin-binding protein 2 (PBP2), are also class A and C β-lactamase inhibitors. To structurally probe their mode of PBP2 inhibition as well as investigate why P. aeruginosa PBP2 is less susceptible to inhibition by β-lactam antibiotics compared to the Escherichia coli PBP2, we determined the crystal structure of P. aeruginosa PBP2 in complex with WCK 5153. WCK 5153 forms an inhibitory covalent bond with the catalytic S327 of PBP2. The structure suggests a significant role for the diacylhydrazide moiety of WCK 5153 in interacting with the aspartate in the S-X-N/D PBP motif. Modeling of zidebactam in the active site of PBP2 reveals a similar binding mode. Both DBOs increase the melting temperature of PBP2, affirming their stabilizing interactions. To aid in the design of DBOs that can inhibit multiple PBPs, the ability of three DBOs to interact with P. aeruginosa PBP3 was explored crystallographically. Even though the DBOs show covalent binding to PBP3, they destabilized PBP3. Overall, the studies provide insights into zidebactam and WCK 5153 inhibition of PBP2 compared to their inhibition of PBP3 and the evolutionarily related KPC-2 β-lactamase. These molecular insights into the dual-target DBOs advance our knowledge regarding further DBO optimization efforts to develop novel potent β-lactamase-resistant, non-β-lactam PBP inhibitors.IMPORTANCE Antibiotic resistance is a significant clinical problem. Developing novel antibiotics that overcome known resistance mechanisms is highly desired. Diazabicyclooctane inhibitors such as zidebactam possess this potential as they readily inactivate penicillin-binding proteins, yet cannot be degraded by β-lactamases. In this study, we characterized the inhibition by diazabicyclooctanes of penicillin-binding proteins PBP2 and PBP3 from Pseudomonas aeruginosa using protein crystallography and biophysical analyses. These structures and analyses help define the antibiotic properties of these inhibitors, explain the decreased susceptibility of P. aeruginosa PBP2 to be inhibited by β-lactam antibiotics, and provide insights that could be used for further antibiotic development.
Keywords: Pseudomonas aeruginosa; antibiotic resistance; penicillin-binding proteins; structural biology.
Figures


Similar articles
-
Molecular Characterization of WCK 5222 (Cefepime/Zidebactam)-Resistant Mutants Developed from a Carbapenem-Resistant Pseudomonas aeruginosa Clinical Isolate.Microbiol Spectr. 2022 Feb 23;10(1):e0267821. doi: 10.1128/spectrum.02678-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196805 Free PMC article.
-
WCK 5107 (Zidebactam) and WCK 5153 Are Novel Inhibitors of PBP2 Showing Potent "β-Lactam Enhancer" Activity against Pseudomonas aeruginosa, Including Multidrug-Resistant Metallo-β-Lactamase-Producing High-Risk Clones.Antimicrob Agents Chemother. 2017 May 24;61(6):e02529-16. doi: 10.1128/AAC.02529-16. Print 2017 Jun. Antimicrob Agents Chemother. 2017. PMID: 28289035 Free PMC article.
-
In Vitro and In Vivo Activities of β-Lactams in Combination with the Novel β-Lactam Enhancers Zidebactam and WCK 5153 against Multidrug-Resistant Metallo-β-Lactamase-Producing Klebsiella pneumoniae.Antimicrob Agents Chemother. 2019 Apr 25;63(5):e00128-19. doi: 10.1128/AAC.00128-19. Print 2019 May. Antimicrob Agents Chemother. 2019. PMID: 30782985 Free PMC article.
-
New Drugs for Multidrug-Resistant Gram-Negative Organisms: Time for Stewardship.Drugs. 2019 May;79(7):705-714. doi: 10.1007/s40265-019-01112-1. Drugs. 2019. PMID: 30972660 Review.
-
Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations.Drugs. 2018 Jan;78(1):65-98. doi: 10.1007/s40265-017-0851-9. Drugs. 2018. PMID: 29230684 Review.
Cited by
-
Molecular Characterization of WCK 5222 (Cefepime/Zidebactam)-Resistant Mutants Developed from a Carbapenem-Resistant Pseudomonas aeruginosa Clinical Isolate.Microbiol Spectr. 2022 Feb 23;10(1):e0267821. doi: 10.1128/spectrum.02678-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196805 Free PMC article.
-
Design and Synthesis of Novel Antimicrobial Agents.Antibiotics (Basel). 2023 Mar 22;12(3):628. doi: 10.3390/antibiotics12030628. Antibiotics (Basel). 2023. PMID: 36978495 Free PMC article. Review.
-
Cefiderocol susceptibility endows hope in treating carbapenem-resistant Pseudomonas aeruginosa: insights from in vitro and in silico evidence.RSC Adv. 2024 Jul 8;14(30):21328-21341. doi: 10.1039/d4ra04302b. eCollection 2024 Jul 5. RSC Adv. 2024. PMID: 38979460 Free PMC article.
-
Drug Discovery in the Field of β-Lactams: An Academic Perspective.Antibiotics (Basel). 2024 Jan 8;13(1):59. doi: 10.3390/antibiotics13010059. Antibiotics (Basel). 2024. PMID: 38247618 Free PMC article. Review.
-
Structural Characterization of the D179N and D179Y Variants of KPC-2 β-Lactamase: Ω-Loop Destabilization as a Mechanism of Resistance to Ceftazidime-Avibactam.Antimicrob Agents Chemother. 2022 Apr 19;66(4):e0241421. doi: 10.1128/aac.02414-21. Epub 2022 Mar 28. Antimicrob Agents Chemother. 2022. PMID: 35341315 Free PMC article.
References
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi:10.1016/S1473-3099(17)30753-3. - DOI - PubMed
-
- Mullane EM, Avery LM, Nicolau DP. 2019. Comparative evaluation of the in vitro activities of WCK 5222 (cefepime-zidebactam) and combination antibiotic therapies against carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 64:e01669-20. doi:10.1128/AAC.01669-19. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

