The Balance of Neutrophil Extracellular Trap Formation and Nuclease Degradation: an Unknown Role of Bacterial Coinfections in COVID-19 Patients?
- PMID: 33593982
- PMCID: PMC8545112
- DOI: 10.1128/mBio.03304-20
The Balance of Neutrophil Extracellular Trap Formation and Nuclease Degradation: an Unknown Role of Bacterial Coinfections in COVID-19 Patients?
Abstract
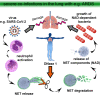
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is leading to public health crises worldwide. An understanding of the pathogenesis and the development of treatment strategies is of high interest. Recently, neutrophil extracellular traps (NETs) have been identified as a potential driver of severe SARS-CoV-2 infections in humans. NETs are extracellular DNA fibers released by neutrophils after contact with various stimuli and accumulate antimicrobial substances or host defense peptides. When massively released, NETs are described to contribute to immunothrombosis in acute respiratory distress syndrome and in vascular occlusions. Based on the increasing evidence that NETs contribute to severe COVID-19 cases, DNase treatment of COVID-19 patients to degrade NETs is widely discussed as a potential therapeutic strategy. Here, we discuss potential detrimental effects of NETs and their nuclease degradation, since NET fragments can boost certain bacterial coinfections and thereby increase the severity of the disease.
Keywords: COVID-19; NETs; neutrophils.
Copyright © 2021 de Buhr and von Köckritz-Blickwede.
Figures
Similar articles
-
Impaired Degradation of Neutrophil Extracellular Traps: A Possible Severity Factor of Elderly Male COVID-19 Patients.J Innate Immun. 2022;14(5):461-476. doi: 10.1159/000521594. Epub 2022 Jan 27. J Innate Immun. 2022. PMID: 35086104 Free PMC article.
-
Significance of NETs Formation in COVID-19.Cells. 2021 Jan 14;10(1):151. doi: 10.3390/cells10010151. Cells. 2021. PMID: 33466589 Free PMC article. Review.
-
Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis.J Clin Invest. 2020 Nov 2;130(11):6151-6157. doi: 10.1172/JCI141374. J Clin Invest. 2020. PMID: 32759504 Free PMC article. Clinical Trial.
-
Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome.Blood. 2020 Sep 3;136(10):1169-1179. doi: 10.1182/blood.2020007008. Blood. 2020. PMID: 32597954 Free PMC article.
-
NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond.Front Immunol. 2022 Mar 2;13:838011. doi: 10.3389/fimmu.2022.838011. eCollection 2022. Front Immunol. 2022. PMID: 35309344 Free PMC article. Review.
Cited by
-
Pattern Recognition Proteins: First Line of Defense Against Coronaviruses.Front Immunol. 2021 Sep 23;12:652252. doi: 10.3389/fimmu.2021.652252. eCollection 2021. Front Immunol. 2021. PMID: 34630377 Free PMC article. Review.
-
Upregulating Human Cathelicidin Antimicrobial Peptide LL-37 Expression May Prevent Severe COVID-19 Inflammatory Responses and Reduce Microthrombosis.Front Immunol. 2022 May 12;13:880961. doi: 10.3389/fimmu.2022.880961. eCollection 2022. Front Immunol. 2022. PMID: 35634307 Free PMC article. Review.
-
Pathogen-Derived Nucleases: An Effective Weapon for Escaping Extracellular Traps.Front Immunol. 2022 Jul 5;13:899890. doi: 10.3389/fimmu.2022.899890. eCollection 2022. Front Immunol. 2022. PMID: 35865526 Free PMC article. Review.
-
Coagulopathies after Vaccination against SARS-CoV-2 May Be Derived from a Combined Effect of SARS-CoV-2 Spike Protein and Adenovirus Vector-Triggered Signaling Pathways.Int J Mol Sci. 2021 Oct 6;22(19):10791. doi: 10.3390/ijms221910791. Int J Mol Sci. 2021. PMID: 34639132 Free PMC article. Review.
-
Risk Factors of Severe COVID-19: A Review of Host, Viral and Environmental Factors.Viruses. 2023 Jan 7;15(1):175. doi: 10.3390/v15010175. Viruses. 2023. PMID: 36680215 Free PMC article. Review.
References
-
- Neumann A, Völlger L, Berends ETM, Molhoek EM, Stapels DAC, Midon M, Friães A, Pingoud A, Rooijakkers SHM, Gallo RL, Mörgelin M, Nizet V, Naim HY, von Köckritz-Blickwede M. 2014. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun 6:860–868. doi:10.1159/000363699. - DOI - PMC - PubMed
-
- de Buhr N, Reuner F, Neumann A, Stump-Guthier C, Tenenbaum T, Schroten H, Ishikawa H, Müller K, Beineke A, Hennig-Pauka I, Gutsmann T, Valentin-Weigand P, Baums CG, von Köckritz-Blickwede M. 2017. Neutrophil extracellular trap formation in the Streptococcus suis-infected cerebrospinal fluid compartment. Cell Microbiol 19:e12649. doi:10.1111/cmi.12649. - DOI - PubMed
-
- Meurer M, Öhlmann S, Bonilla MC, Valentin-Weigand P, Beineke A, Hennig-Pauka I, Schwerk C, Schroten H, Baums CG, von Köckritz-Blickwede M, de Buhr N. 2020. Role of bacterial and host DNases on host-pathogen interaction during Streptococcus suis meningitis. Int J Mol Sci 21:5289. doi:10.3390/ijms21155289. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

