Asymmetric opening of the homopentameric 5-HT3A serotonin receptor in lipid bilayers
- PMID: 33594077
- PMCID: PMC7887223
- DOI: 10.1038/s41467-021-21016-7
Asymmetric opening of the homopentameric 5-HT3A serotonin receptor in lipid bilayers
Abstract
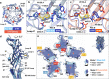
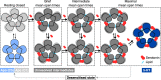
Pentameric ligand-gated ion channels (pLGICs) of the Cys-loop receptor family are key players in fast signal transduction throughout the nervous system. They have been shown to be modulated by the lipid environment, however the underlying mechanism is not well understood. We report three structures of the Cys-loop 5-HT3A serotonin receptor (5HT3R) reconstituted into saposin-based lipid bilayer discs: a symmetric and an asymmetric apo state, and an asymmetric agonist-bound state. In comparison to previously published 5HT3R conformations in detergent, the lipid bilayer stabilises the receptor in a more tightly packed, 'coupled' state, involving a cluster of highly conserved residues. In consequence, the agonist-bound receptor conformation adopts a wide-open pore capable of conducting sodium ions in unbiased molecular dynamics (MD) simulations. Taken together, we provide a structural basis for the modulation of 5HT3R by the membrane environment, and a model for asymmetric activation of the receptor.
Conflict of interest statement
H.V. and S.Y. are cofounders of AlphaMol Science Ltd. The remaining authors declare no competing interests.
Figures







Similar articles
-
Structure of tetrameric forms of the serotonin-gated 5-HT3A receptor ion channel.EMBO J. 2024 Oct;43(20):4451-4471. doi: 10.1038/s44318-024-00191-5. Epub 2024 Sep 4. EMBO J. 2024. PMID: 39232129 Free PMC article.
-
Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor.Nature. 2018 Nov;563(7730):270-274. doi: 10.1038/s41586-018-0660-7. Epub 2018 Oct 31. Nature. 2018. PMID: 30401837 Free PMC article.
-
Two-dimensional crystallization of the mouse serotonin 5-HT3A receptor.Micron. 2017 Jan;92:19-24. doi: 10.1016/j.micron.2016.10.004. Epub 2016 Oct 22. Micron. 2017. PMID: 27825023
-
Ion channels gated by acetylcholine and serotonin: structures, biology, and drug discovery.Acta Pharmacol Sin. 2015 Aug;36(8):895-907. doi: 10.1038/aps.2015.66. Acta Pharmacol Sin. 2015. PMID: 26238288 Free PMC article. Review.
-
The 5-hydroxytryptamine type 3 (5-HT3) receptor reveals a novel determinant of single-channel conductance.Biochem Soc Trans. 2004 Jun;32(Pt3):547-52. doi: 10.1042/BST0320547. Biochem Soc Trans. 2004. PMID: 15157181 Review.
Cited by
-
Symmetry-adapted Markov state models of closing, opening, and desensitizing in α 7 nicotinic acetylcholine receptors.Nat Commun. 2024 Oct 18;15(1):9022. doi: 10.1038/s41467-024-53170-z. Nat Commun. 2024. PMID: 39424796 Free PMC article.
-
Phylogenetic analyses of 5-hydroxytryptamine 3 (5-HT3) receptors in Metazoa.PLoS One. 2023 Mar 1;18(3):e0281507. doi: 10.1371/journal.pone.0281507. eCollection 2023. PLoS One. 2023. PMID: 36857360 Free PMC article.
-
Cryo-EM structures of prokaryotic ligand-gated ion channel GLIC provide insights into gating in a lipid environment.Nat Commun. 2024 Apr 5;15(1):2967. doi: 10.1038/s41467-024-47370-w. Nat Commun. 2024. PMID: 38580666 Free PMC article.
-
Structure of tetrameric forms of the serotonin-gated 5-HT3A receptor ion channel.EMBO J. 2024 Oct;43(20):4451-4471. doi: 10.1038/s44318-024-00191-5. Epub 2024 Sep 4. EMBO J. 2024. PMID: 39232129 Free PMC article.
-
Computational drug development for membrane protein targets.Nat Biotechnol. 2024 Feb;42(2):229-242. doi: 10.1038/s41587-023-01987-2. Epub 2024 Feb 15. Nat Biotechnol. 2024. PMID: 38361054 Review.
References
-
- Criado M, Eibl H, Barrantes F. Functional properties of the acetylcholine receptor incorporated in model lipid membranes. Differential effects of chain length and head group of phospholipids on receptor affinity states and receptor-mediated ion translocation. J. Biol. Chem. 1984;259:9188–9198. doi: 10.1016/S0021-9258(17)47283-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

