Pancreatic glycoprotein 2 is a first line of defense for mucosal protection in intestinal inflammation
- PMID: 33594081
- PMCID: PMC7887276
- DOI: 10.1038/s41467-021-21277-2
Pancreatic glycoprotein 2 is a first line of defense for mucosal protection in intestinal inflammation
Abstract
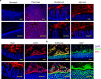
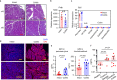
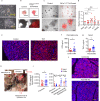
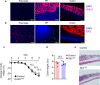
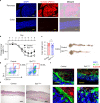
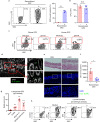
Increases in adhesive and invasive commensal bacteria, such as Escherichia coli, and subsequent disruption of the epithelial barrier is implicated in the pathogenesis of inflammatory bowel disease (IBD). However, the protective systems against such barrier disruption are not fully understood. Here, we show that secretion of luminal glycoprotein 2 (GP2) from pancreatic acinar cells is induced in a TNF-dependent manner in mice with chemically induced colitis. Fecal GP2 concentration is also increased in Crohn's diease patients. Furthermore, pancreas-specific GP2-deficient colitis mice have more severe intestinal inflammation and a larger mucosal E. coli population than do intact mice, indicating that digestive-tract GP2 binds commensal E. coli, preventing epithelial attachment and penetration. Thus, the pancreas-intestinal barrier axis and pancreatic GP2 are important as a first line of defense against adhesive and invasive commensal bacteria during intestinal inflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

