The Prospective Dutch Colorectal Cancer (PLCRC) cohort: real-world data facilitating research and clinical care
- PMID: 33594104
- PMCID: PMC7887218
- DOI: 10.1038/s41598-020-79890-y
The Prospective Dutch Colorectal Cancer (PLCRC) cohort: real-world data facilitating research and clinical care
Abstract
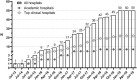
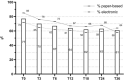
Real-world data (RWD) sources are important to advance clinical oncology research and evaluate treatments in daily practice. Since 2013, the Prospective Dutch Colorectal Cancer (PLCRC) cohort, linked to the Netherlands Cancer Registry, serves as an infrastructure for scientific research collecting additional patient-reported outcomes (PRO) and biospecimens. Here we report on cohort developments and investigate to what extent PLCRC reflects the "real-world". Clinical and demographic characteristics of PLCRC participants were compared with the general Dutch CRC population (n = 74,692, Dutch-ref). To study representativeness, standardized differences between PLCRC and Dutch-ref were calculated, and logistic regression models were evaluated on their ability to distinguish cohort participants from the Dutch-ref (AU-ROC 0.5 = preferred, implying participation independent of patient characteristics). Stratified analyses by stage and time-period (2013-2016 and 2017-Aug 2019) were performed to study the evolution towards RWD. In August 2019, 5744 patients were enrolled. Enrollment increased steeply, from 129 participants (1 hospital) in 2013 to 2136 (50 of 75 Dutch hospitals) in 2018. Low AU-ROC (0.65, 95% CI: 0.64-0.65) indicates limited ability to distinguish cohort participants from the Dutch-ref. Characteristics that remained imbalanced in the period 2017-Aug'19 compared with the Dutch-ref were age (65.0 years in PLCRC, 69.3 in the Dutch-ref) and tumor stage (40% stage-III in PLCRC, 30% in the Dutch-ref). PLCRC approaches to represent the Dutch CRC population and will ultimately meet the current demand for high-quality RWD. Efforts are ongoing to improve multidisciplinary recruitment which will further enhance PLCRC's representativeness and its contribution to a learning healthcare system.
Conflict of interest statement
MK reports institutional financial interests with Amgen, Bayer, BMS, Merck-Serono, Nordic Pharma, Roche, Servier, Sirtex, and Sanofi-Aventis. MK reports the following non-financial interests: an advisory role for ZON-MW, membership of the scientific board of the Dutch Cancer Society (KWF), chairmanship of the Dutch Colorectal Cancer Group (DCCG), principal investigator (PI) of the Prospective Dutch Colorectal Cancer (PLCRC) cohort. GRV reports institutional financial interests with Servier, Merck, Bayer, Sirtex, BMS, and Lilly. JMLR reports institutional financial interests with Servier, Merck, and Bayer. PDS reports institutional financial interests with The eNose Company, Norgine, and Motus GI. JWGD, MAGE, HMV, WMUG, and AMM report no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

