Effects of open-label placebos in clinical trials: a systematic review and meta-analysis
- PMID: 33594150
- PMCID: PMC7887232
- DOI: 10.1038/s41598-021-83148-6
Effects of open-label placebos in clinical trials: a systematic review and meta-analysis
Erratum in
-
Author Correction: Effects of open-label placebos in clinical trials: a systematic review and meta-analysis.Sci Rep. 2021 Aug 25;11(1):17436. doi: 10.1038/s41598-021-96604-0. Sci Rep. 2021. PMID: 34433888 Free PMC article. No abstract available.
Abstract
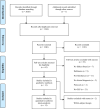
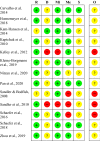
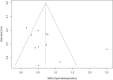
Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze the effect of OLPs in comparison to no treatment in clinical trials. A systematic literature search was carried out in February 2020. Randomized controlled trials of any medical condition or mental disorder comparing OLPs to no treatment were included. Data extraction and risk of bias rating were independently assessed. 1246 records were screened and thirteen studies were included into the systematic review. Eleven trials were eligible for meta-analysis. These trials assessed effects of OLPs on back pain, cancer-related fatigue, attention deficit hyperactivity disorder, allergic rhinitis, major depression, irritable bowel syndrome and menopausal hot flushes. Risk of bias was moderate among all studies. We found a significant overall effect (standardized mean difference = 0.72, 95% Cl 0.39-1.05, p < 0.0001, I2 = 76%) of OLP. Thus, OLPs appear to be a promising treatment in different conditions but the respective research is in its infancy. More research is needed, especially with respect to different medical and mental disorders and instructions accompanying the OLP administration as well as the role of expectations and mindsets.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu. Rev. Psychol. 2008;59:565–590. - PubMed
-
- Beecher HK. The powerful placebo. JAMA. 1955;159:1602–1606. - PubMed
-
- Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2008;67:1716–1723. - PubMed
-
- Kaptchuk TJ, Miller FG. Placebo effects in medicine. N. Engl. J. Med. 2015;373:8–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

