Formation of interstellar cyanoacetamide: a rotational and computational study
- PMID: 33594291
- PMCID: PMC7116755
- DOI: 10.1051/0004-6361/202038766
Formation of interstellar cyanoacetamide: a rotational and computational study
Abstract
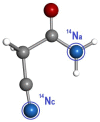
Context: Cyanoacetamide is a -CN bearing molecule that is also an amide derivative target molecule in the interstellar medium.
Aims: The aim of our investigation is to analyze the feasibility of a plausible formation process of protonated cyanoacetamide under interstellar conditions and to provide direct experimental frequencies of the ground vibrational state of the neutral form in the microwave region in order to enable its eventual identification in the interstellar medium.
Methods: We used high-level theoretical computations to study the formation process of protonated cyanoacetamide. Furthermore, we employed a high-resolution laser-ablation molecular beam Fourier transform spectroscopic technique to measure the frequencies of the neutral form.
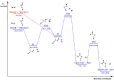
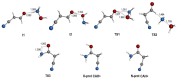
Results: We report the first rotational characterization of cyanoacetamide, and a precise set of the relevant rotational spectroscopic constants have been determined as a first step to identifying the molecule in the interstellar medium. We fully explored the potential energy surface to study a gas-phase reaction on the formation process of protonated cyanoacetamide. We found that an exothermic process with no net activation barrier is initiated by the high-energy isomer of protonated hydroxylamine, which leads to protonated cyanoacetamide.
Keywords: ISM: molecules; astrochemistry; molecular data; molecular processes.
Figures

References
-
- Alonso JL, López JC. Gas-Phase IR Spectroscopy and Structure of Biological Molecules. Vol. 335 Cham: Springer International Publishing; 2015.
-
- Alonso JL, Pérez C, Eugenia Sanz M, et al. Phys Chem Chem Phys. 2009;11:617. - PubMed
-
- Alonso JL, Lozoya MAI, Peña, López JC, et al. Chem Sci. 2014;5:515.
-
- Alonso ER, Kolesniková L, Alonso JL. J Chem Phys. 2017;147 124312. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
