Berberine protects human and rat cardiomyocytes from hypoxia/reoxygenation-triggered apoptosis
- PMID: 33594316
- PMCID: PMC7868847
Berberine protects human and rat cardiomyocytes from hypoxia/reoxygenation-triggered apoptosis
Retraction in
-
Berberine protects human and rat cardiomyocytes from hypoxia/reoxygenation-triggered apoptosis [Retraction].Am J Transl Res. 2021 Dec 15;13(12):14241. eCollection 2021. Am J Transl Res. 2021. PMID: 35035773 Free PMC article.
Abstract
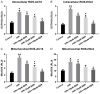
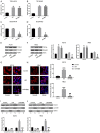
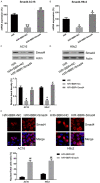
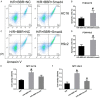
Berberine (BBR) confers potential cardioprotective effects. However, the relevant mechanisms underlying its regulation of cardiomyocyte survival following hypoxia/reoxygenation (H/R) treatment remain unknown. The present study investigated whether BBR could protect H/R by suppressing apoptosis and explored how TGF-β/Smad4 signaling pathway influenced H/R in vitro. Two cardiomyocyte cell lines-AC16 and H9c2- were treated with H/R and BBR. The survival and apoptosis of these two cell lines were assessed using the MTT and BrdU assays and western blotting (WB) and flow cytometry. Mitochondrial reactive oxygen species (ROS) and caspase (Cas)-3, Cas-8, and Cas-9 activation were evaluated using enzyme-linked immunosorbent assay as well as WB. Compared to the control group, H/R resulted in notable cell apoptosis, whereas BBR treatment evidently counteracted the process. BBR also markedly suppressed H/R-triggered excessive mitochondrial ROS generation and inhibited Smad4 expression. Overexpressing Smad4 in BBR-treated H/R-exposed cardiomyocytes reversed the effect of BBR treatment on apoptosis. Therefore, BBR protects H/R-treated cardiomyocytes from apoptosis by inhibiting the TGF-β/Smad4 signaling pathway.
Keywords: BBR; TGF-β/smad4 pathway; apoptosis; cardiomyocytes; hypoxia/reoxygenation.
AJTR Copyright © 2021.
Conflict of interest statement
None.
Figures


Similar articles
-
Berberine protects renal tubular cells against hypoxia/reoxygenation injury via the Sirt1/p53 pathway.J Nat Med. 2018 Jun;72(3):715-723. doi: 10.1007/s11418-018-1210-1. Epub 2018 Apr 21. J Nat Med. 2018. PMID: 29680964
-
Protective effect of berberine against cardiac ischemia/reperfusion injury by inhibiting apoptosis through the activation of Smad7.Mol Cell Probes. 2018 Apr;38:38-44. doi: 10.1016/j.mcp.2017.12.002. Epub 2017 Dec 24. Mol Cell Probes. 2018. PMID: 29278748
-
Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways.J Transl Med. 2013 Jan 29;11:24. doi: 10.1186/1479-5876-11-24. J Transl Med. 2013. PMID: 23360542 Free PMC article.
-
Berberine Protects Against Simulated Ischemia/Reperfusion Injury-Induced H9C2 Cardiomyocytes Apoptosis In Vitro and Myocardial Ischemia/Reperfusion-Induced Apoptosis In Vivo by Regulating the Mitophagy-Mediated HIF-1α/BNIP3 Pathway.Front Pharmacol. 2020 Mar 27;11:367. doi: 10.3389/fphar.2020.00367. eCollection 2020. Front Pharmacol. 2020. PMID: 32292345 Free PMC article.
-
Clematichinenoside (AR) Attenuates Hypoxia/Reoxygenation-Induced H9c2 Cardiomyocyte Apoptosis via a Mitochondria-Mediated Signaling Pathway.Molecules. 2016 May 30;21(6):683. doi: 10.3390/molecules21060683. Molecules. 2016. PMID: 27248986 Free PMC article.
References
-
- Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–323. - PubMed
-
- Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. - PubMed
-
- Remmers DE, Wang P, Cioffi WG, Bland KI, Chaudry IH. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919–H2925. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
