A multi-omics-based serial deep learning approach to predict clinical outcomes of single-agent anti-PD-1/PD-L1 immunotherapy in advanced stage non-small-cell lung cancer
- PMID: 33594323
- PMCID: PMC7868825
A multi-omics-based serial deep learning approach to predict clinical outcomes of single-agent anti-PD-1/PD-L1 immunotherapy in advanced stage non-small-cell lung cancer
Abstract
Only 20% NSCLC patients benefit from immunotherapy with a durable response. Current biomarkers are limited by the availability of samples and do not accurately predict who will benefit from immunotherapy. To develop a unified deep learning model to integrate multimodal serial information from CT with laboratory and baseline clinical information. We retrospectively analyzed 1633 CT scans and 3414 blood samples from 200 advanced stage NSCLC patients who received single anti-PD-1/PD-L1 agent between April 2016 and December 2019. Multidimensional information, including serial radiomics, laboratory data and baseline clinical data, was used to develop and validate deep learning models to identify immunotherapy responders and nonresponders. A Simple Temporal Attention (SimTA) module was developed to process asynchronous time-series imaging and laboratory data. Using cross-validation, the 90-day deep learning-based predicting model showed a good performance in distinguishing responders from nonresponders, with an area under the curve (AUC) of 0.80 (95% CI: 0.74-0.86). Before immunotherapy, we stratified the patients into high- and low-risk nonresponders using the model. The low-risk group had significantly longer progression-free survival (PFS) (8.4 months, 95% CI: 5.49-11.31 vs. 1.5 months, 95% CI: 1.29-1.71; HR 3.14, 95% CI: 2.27-4.33; log-rank test, P<0.01) and overall survival (OS) (26.7 months, 95% CI: 18.76-34.64 vs. 8.6 months, 95% CI: 4.55-12.65; HR 2.46, 95% CI: 1.73-3.51; log-rank test, P<0.01) than the high-risk group. An exploratory analysis of 93 patients with stable disease (SD) [after first efficacy assessment according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1] also showed that the 90-day model had a good prediction of survival and low-risk patients had significantly longer PFS (11.1 months, 95% CI: 10.24-11.96 vs. 3.3 months, 95% CI: 0.34-6.26; HR 2.93, 95% CI: 1.69-5.10; log-rank test, P<0.01) and OS (31.7 months, 95% CI: 23.64-39.76 vs. 17.2 months, 95% CI: 7.22-27.18; HR 2.22, 95% CI: 1.17-4.20; log-rank test, P=0.01) than high-risk patients. In conclusion, the SimTA-based multi-omics serial deep learning provides a promising methodology for predicting response of advanced NSCLC patients to anti-PD-1/PD-L1 monotherapy. Moreover, our model could better differentiate survival benefit among SD patients than the traditional RECIST evaluation method.
Keywords: NSCLC; SimTA; multi-omics serial deep learning.
AJTR Copyright © 2021.
Conflict of interest statement
None.
Figures
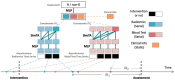
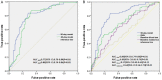
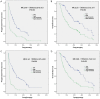
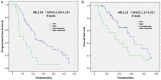
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. - PubMed
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. - PubMed
-
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. - PMC - PubMed
-
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
