Multi-scale optoacoustic molecular imaging of brain diseases
- PMID: 33594473
- PMCID: PMC8566397
- DOI: 10.1007/s00259-021-05207-4
Multi-scale optoacoustic molecular imaging of brain diseases
Abstract
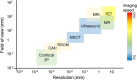
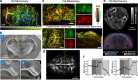
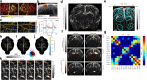
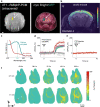
The ability to non-invasively visualize endogenous chromophores and exogenous probes and sensors across the entire rodent brain with the high spatial and temporal resolution has empowered optoacoustic imaging modalities with unprecedented capacities for interrogating the brain under physiological and diseased conditions. This has rapidly transformed optoacoustic microscopy (OAM) and multi-spectral optoacoustic tomography (MSOT) into emerging research tools to study animal models of brain diseases. In this review, we describe the principles of optoacoustic imaging and showcase recent technical advances that enable high-resolution real-time brain observations in preclinical models. In addition, advanced molecular probe designs allow for efficient visualization of pathophysiological processes playing a central role in a variety of neurodegenerative diseases, brain tumors, and stroke. We describe outstanding challenges in optoacoustic imaging methodologies and propose a future outlook.
Keywords: Brain; Multi-spectral optoacoustic tomography (MSOT); Neuroimaging; Optical imaging; Photoacoustics.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

