Calcium Dynamics and Water Transport in Salivary Acinar Cells
- PMID: 33594615
- PMCID: PMC8018713
- DOI: 10.1007/s11538-020-00841-9
Calcium Dynamics and Water Transport in Salivary Acinar Cells
Abstract
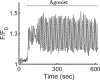
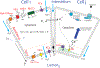
Saliva is secreted from the acinar cells of the salivary glands, using mechanisms that are similar to other types of water-transporting epithelial cells. Using a combination of theoretical and experimental techniques, over the past 20 years we have continually developed and modified a quantitative model of saliva secretion, and how it is controlled by the dynamics of intracellular calcium. However, over approximately the past 5 years there have been significant developments both in our understanding of the underlying mechanisms and in the way these mechanisms should best be modelled. Here, we review the traditional understanding of how saliva is secreted, and describe how our work has suggested important modifications to this traditional view. We end with a brief description of the most recent data from living animals and discuss how this is now contributing to yet another iteration of model construction and experimental investigation.
Keywords: Calcium oscillations; Mathematical modelling; Saliva secretion; Three-dimensional finite element computations; Water transport.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Figures










References
-
- Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, Chiorini JA, Voutetakis A, Leakan RA, Van Waes C, Mitchell JB, Delporte C, Wang S, Kaminsky SM, Illei GG (2006) Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta 1758(8):1071–7, DOI 10.1016/j.bbamem.2005.11.006 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

