Randomized, Double-Blind, Placebo-Controlled Phase II Study of Yeast-Brachyury Vaccine (GI-6301) in Combination with Standard-of-Care Radiotherapy in Locally Advanced, Unresectable Chordoma
- PMID: 33594772
- PMCID: PMC8100546
- DOI: 10.1002/onco.13720
Randomized, Double-Blind, Placebo-Controlled Phase II Study of Yeast-Brachyury Vaccine (GI-6301) in Combination with Standard-of-Care Radiotherapy in Locally Advanced, Unresectable Chordoma
Abstract
Background: Brachyury is a transcription factor overexpressed in chordoma and is associated with chemotherapy resistance and epithelial-to-mesenchymal transition. GI-6301 is a recombinant, heat-killed Saccharomyces cerevisiae yeast-based vaccine targeting brachyury. A previous phase I trial of GI-6301 demonstrated a signal of clinical activity in chordomas. This trial evaluated synergistic effects of GI-6301 vaccine plus radiation.
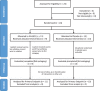
Materials and methods: Adults with locally advanced, unresectable chordoma were treated on a randomized, placebo-controlled trial. Patients received three doses of GI-6301 (80 × 107 yeast cells) or placebo followed by radiation, followed by continued vaccine or placebo until progression. Primary endpoint was overall response rate, defined as a complete response (CR) or partial response (PR) in the irradiated tumor site at 24 months. Immune assays were conducted to evaluate immunogenicity.
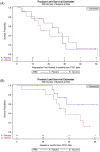
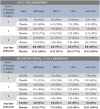
Results: Between May 2015 and September 2019, 24 patients enrolled on the first randomized phase II study in chordoma. There was one PR in each arm; no CRs were observed. Median progressive-free survival for vaccine and placebo arms was 20.6 months (95% confidence interval [CI], 5.7-37.5 months) and 25.9 months (95% CI, 9.2-30.8 months), respectively. Hazard ratio was 1.02 (95% CI, 0.38-2.71). Vaccine was well tolerated with no vaccine-related serious adverse events. Preexisting brachyury-specific T cells were detected in most patients in both arms. Most patients developed T-cell responses during therapy, with no difference between arms in frequency or magnitude of response.
Conclusion: No difference in overall response rate was observed, leading to early discontinuation of this trial due to low conditional power to detect statistical difference at the planned end of accrual.
Implications for practice: Chordoma is a rare neoplasm lacking effective systemic therapies for advanced, unresectable disease. Lack of clinically actionable somatic mutations in chordoma makes development of targeted therapy quite challenging. While the combination of yeast-brachyury vaccine (GI-6301) and standard radiation therapy did not demonstrate synergistic antitumor effects, brachyury still remains a good target for developmental therapeutics in chordoma. Patients and their oncologists should consider early referral to centers with expertise in chordoma (or sarcoma) and encourage participation in clinical trials.
Keywords: Chordoma; Immunotherapy; Radiation therapy; Randomized clinical trial; Therapeutic vaccine.
Published 2021. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Figures
References
-
- McMaster ML, Goldstein AM, Bromley CM et al. Chordoma: Incidence and survival patterns in the United States, 1973‐1995. Cancer Causes Control 2001;12:1–11. - PubMed
-
- Chambers KJ, Lin DT, Meier J et al. Incidence and survival patterns of cranial chordoma in the United States. Laryngoscope 2014;124:1097–1102. - PubMed
-
- Vujovic S, Henderson S, Presneau N et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol 2006;209:157–165. - PubMed
-
- Tirabosco R, Mangham DC, Rosenberg AE et al. Brachyury expression in extra‐axial skeletal and soft tissue chordomas: A marker that distinguishes chordoma from mixed tumor/myoepithelioma/parachordoma in soft tissue. Am J Surg Pathol 2008;32:572–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

