Droplet digital PCR of tumor suppressor gene methylation in serial oral rinses of patients with head and neck squamous cell carcinoma
- PMID: 33594807
- PMCID: PMC8248028
- DOI: 10.1002/hed.26647
Droplet digital PCR of tumor suppressor gene methylation in serial oral rinses of patients with head and neck squamous cell carcinoma
Abstract
Background: Head and neck squamous cell carcinoma (HNSCC) currently lacks sensitive approaches to detect cancer-related traits in body fluid.
Methods: Methylation of tumor suppressor genes (TSGs) (PAX5, EDNRB, and DCC) were measured in the oral rinses from 50 HNSCC and 58 control subjects using droplet digital PCR (ddPCR). Diagnostic accuracies in detecting HNSCC and the detection rate of recurrence in the post-treatment monitoring were analyzed.
Results: ddPCR TSG methylation detection in oral rinses for diagnosis of HNSCC had an AUC of 0.892 for PAX5, 0.753 for EDNRB, and 0.729 for DCC. Significant drop of TSG methylation was observed after completion of surgery (p < 0.01). 76.9% of the relapse cases had a pre-emptive rebound of methylation above presurgery levels in at least one of the tested markers before confirmed recurrence.
Conclusions: Utilizing ddPCR for TSG methylation detection in oral rinses shows potential for detection and monitoring of HNSCC.
Keywords: droplet digital PCR; head and neck cancer; head and neck squamous cell carcinoma; liquid biopsy; methylation.
© 2021 The Authors. Head & Neck published by Wiley Periodicals LLC.
Figures

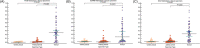



Similar articles
-
Evaluation of DNA methylation levels of SEPT9 and SHOX2 in plasma of patients with head and neck squamous cell carcinoma using droplet digital PCR.Oncol Rep. 2024 Mar;51(3):52. doi: 10.3892/or.2024.8711. Epub 2024 Feb 1. Oncol Rep. 2024. PMID: 38299234 Free PMC article.
-
KIF1A and EDNRB are differentially methylated in primary HNSCC and salivary rinses.Int J Cancer. 2010 Nov 15;127(10):2351-9. doi: 10.1002/ijc.25248. Int J Cancer. 2010. PMID: 20162572 Free PMC article.
-
Correlation of gene methylation in surgical margin imprints with locoregional recurrence in head and neck squamous cell carcinoma.Cancer. 2015 Jun 15;121(12):1957-65. doi: 10.1002/cncr.29303. Epub 2015 Mar 13. Cancer. 2015. PMID: 25773145 Free PMC article.
-
DNA methylation in tumour and normal mucosal tissue of head and neck squamous cell carcinoma (HNSCC) patients: new diagnostic approaches and treatment.Med Oncol. 2013;30(3):654. doi: 10.1007/s12032-013-0654-0. Epub 2013 Jul 4. Med Oncol. 2013. PMID: 23824644 Review.
-
DNA Methylation as a Diagnostic, Prognostic, and Predictive Biomarker in Head and Neck Cancer.Int J Mol Sci. 2023 Feb 3;24(3):2996. doi: 10.3390/ijms24032996. Int J Mol Sci. 2023. PMID: 36769317 Free PMC article. Review.
Cited by
-
Comparative Analysis of Two Digital PCR Platforms for Detecting DNA Methylation in Patient Samples.Cell Biochem Funct. 2025 Aug;43(8):e70112. doi: 10.1002/cbf.70112. Cell Biochem Funct. 2025. PMID: 40826981 Free PMC article.
-
Personalized ctDNA for Monitoring Disease Status in Head and Neck Squamous Cell Carcinoma.Clin Cancer Res. 2024 Aug 1;30(15):3329-3336. doi: 10.1158/1078-0432.CCR-24-0590. Clin Cancer Res. 2024. PMID: 38824449 Free PMC article.
-
Evaluation of DNA methylation levels of SEPT9 and SHOX2 in plasma of patients with head and neck squamous cell carcinoma using droplet digital PCR.Oncol Rep. 2024 Mar;51(3):52. doi: 10.3892/or.2024.8711. Epub 2024 Feb 1. Oncol Rep. 2024. PMID: 38299234 Free PMC article.
-
Recent Advancements in the Application of Circulating Tumor DNA as Biomarkers for Early Detection of Cancers.ACS Biomater Sci Eng. 2024 Aug 12;10(8):4740-4756. doi: 10.1021/acsbiomaterials.4c00606. Epub 2024 Jul 1. ACS Biomater Sci Eng. 2024. PMID: 38950521 Free PMC article. Review.
-
Circulating Tumor DNA in Head and Neck Squamous Cell Carcinoma.Cancers (Basel). 2023 Mar 30;15(7):2051. doi: 10.3390/cancers15072051. Cancers (Basel). 2023. PMID: 37046721 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Economopoulou P, Psyrri A. Epidemiology, risk factors and pathogenesis of squamous cell tumours. In: Licitra L, Karamouzis MV, Ghielmini M, eds. Essentials for Clinicians: Head & Neck Cancers. Lugano, Switzerland: European Society for Medical Oncology; 2017:1‐2.
-
- Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91:386‐396. - PubMed
-
- Chan JYK, Tsang RK, Holsinger FC, et al. Prospective clinical trial to evaluate safety and feasibility of using a single port flexible robotic system for transoral head and neck surgery. Oral Oncol. 2019;94:101‐105. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

