Molecular response and quality of life in chronic myeloid leukemia patients treated with intermittent TKIs: First interim analysis of OPTkIMA study
- PMID: 33594821
- PMCID: PMC7940223
- DOI: 10.1002/cam4.3778
Molecular response and quality of life in chronic myeloid leukemia patients treated with intermittent TKIs: First interim analysis of OPTkIMA study
Erratum in
-
Corrigendum.Cancer Med. 2021 May;10(10):3486. doi: 10.1002/cam4.3922. Epub 2021 May 2. Cancer Med. 2021. PMID: 33934555 Free PMC article. No abstract available.
Abstract
Background: Intermittent treatment with TKIs is an option for the great majority (70%-80%) of CML patients who do not achieve a stable deep molecular response and are not eligible for treatment discontinuation. For these patients, the only alternative is to assume TKI continuously, lifelong.
Methods: The Italian phase III multicentric randomized OPTkIMA study started in 2015, with the aim to evaluate if a progressive de-escalation of TKIs (imatinib, nilotinib, and dasatinib) is able to maintain the molecular response (MR3.0 ) and to improve Health Related Quality of Life (HRQoL).
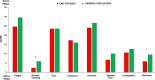
Results: Up to December 2018, 166/185 (90%) elderly CML patients in stable MR3.0 /MR4.0 completed the first year of any TKI intermittent schedule 1 month ON and 1 month OFF. The first year probability of maintaining the MR3.0 was 81% and 23.5% of the patients who lost the molecular response regained the MR3.0 after resuming TKI continuously. Patients' HRQoL at baseline was better than that of matched peers from healthy population. Women was the only factor independently associated with worse baseline HRQoL (p > 0.0001). Overall, global HRQoL worsened at 6 (p < 0.001) but returned to the baseline value at 12 months and it was statistically significantly worse in women (p = 0.001).
Conclusions: De-escalation of any TKI by 1 month ON/OFF schedule maintains the MR3.0 /MR4.0 in 81% of the patients during the first 12-24 months. No patients progressed to accelerated/blastic phase, all the patients (23.5%) losing MR3.0 regained the MR3.0 and none suffered from TKI withdrawn syndrome. The study firstly report on HRQoL in elderly CML patients moving from a continuous daily therapy to a de-escalated intermittent treatment.
Keywords: chronic myeloid leukaemia; intermittent; quality of life; tyrosine kinase inhibitor.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
AI—speaker honoraria from Novartis, Pfizer, and Incyte; EA—consultancy and advisory for Novartis, Bristol Myers Squibb, Incyte, and Pfizer; GR—speaker honoraria from Novartis, Pfizer, and Incyte; MB—consultant and receiving honoraria from Novartis, Incyte, and Takeda; DR—speaker honoraria: MSD, Novartis, Gilead; advisory committees: MSD, Janssen, Gilead. All the other authors declare no conflicts of interest.
Figures




References
-
- Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851‐2857. - PubMed
-
- Rousselot P, Charbonnier A, Cony‐Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic‐phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424‐430. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

