Phenolic-enabled nanotechnology: versatile particle engineering for biomedicine
- PMID: 33595004
- PMCID: PMC8106539
- DOI: 10.1039/d0cs00908c
Phenolic-enabled nanotechnology: versatile particle engineering for biomedicine
Abstract
Phenolics are ubiquitous in nature and have gained immense research attention because of their unique physiochemical properties and widespread industrial use. In recent decades, their accessibility, versatile reactivity, and relative biocompatibility have catalysed research in phenolic-enabled nanotechnology (PEN) particularly for biomedical applications which have been a major benefactor of this emergence, as largely demonstrated by polydopamine and polyphenols. Therefore, it is imperative to overveiw the fundamental mechanisms and synthetic strategies of PEN for state-of-the-art biomedical applications and provide a timely and comprehensive summary. In this review, we will focus on the principles and strategies involved in PEN and summarize the use of the PEN synthetic toolkit for particle engineering and the bottom-up synthesis of nanohybrid materials. Specifically, we will discuss the attractive forces between phenolics and complementary structural motifs in confined particle systems to synthesize high-quality products with controllable size, shape, composition, as well as surface chemistry and function. Additionally, phenolic's numerous applications in biosensing, bioimaging, and disease treatment will be highlighted. This review aims to provide guidelines for new scientists in the field and serve as an up-to-date compilation of what has been achieved in this area, while offering expert perspectives on PEN's use in translational research.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures




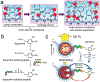

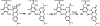




















References
-
- Bravo L, Nutr. Rev, 1998, 56, 317–333. - PubMed
-
- Quideau S, Deffieux D, Douat-Casassus C and Pouysegu L, Angew. Chem., Int. Ed, 2011, 50, 586–621. - PubMed
-
- Liu Y, Ai K and Lu L, Chem. Rev, 2014, 114, 5057–5115. - PubMed
-
- He LR, Mu CD, Shi JB, Zhang QA, Shi B and Lin W, Int. J. Biol. Macromol, 2011, 48, 354–359. - PubMed
-
- Waite JH and Tanzer ML, Science, 1981, 212, 1038–1040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

