Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: First revision
- PMID: 33595124
- PMCID: PMC8252715
- DOI: 10.1002/jimd.12370
Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: First revision
Erratum in
-
Corrigendum.J Inherit Metab Dis. 2022 Jul;45(4):862. doi: 10.1002/jimd.12535. J Inherit Metab Dis. 2022. PMID: 35841205 Free PMC article. No abstract available.
Abstract
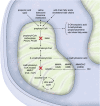
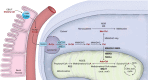
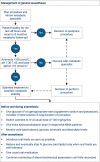
Isolated methylmalonic acidaemia (MMA) and propionic acidaemia (PA) are rare inherited metabolic diseases. Six years ago, a detailed evaluation of the available evidence on diagnosis and management of these disorders has been published for the first time. The article received considerable attention, illustrating the importance of an expert panel to evaluate and compile recommendations to guide rare disease patient care. Since that time, a growing body of evidence on transplant outcomes in MMA and PA patients and use of precursor free amino acid mixtures allows for updates of the guidelines. In this article, we aim to incorporate this newly published knowledge and provide a revised version of the guidelines. The analysis was performed by a panel of multidisciplinary health care experts, who followed an updated guideline development methodology (GRADE). Hence, the full body of evidence up until autumn 2019 was re-evaluated, analysed and graded. As a result, 21 updated recommendations were compiled in a more concise paper with a focus on the existing evidence to enable well-informed decisions in the context of MMA and PA patient care.
Keywords: diagnosis and management; guidelines; inherited metabolic disease; methylmalonic acidaemia; propionic acidaemia.
© 2021 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
D. B. participated in European Metabolic Group meetings sponsored by Nutricia and received research funds from Nutricia. S. C. G. has received a research fund from Nutricia as well as reimbursement for attending a symposium by MetaX and Vitaflo and fees for speaking on educational events from Nutricia and Vitaflo. J. O. S. received institutional honoraria from Swedish Orphan Biovitrum und Immedica Pharma AB. S. S.‐B. has received reimbursement for symposia fees from Nutricia and Dr Schär and research funds from Nutricia. M. R. B. received a research fund from Nutricia and is a member of the clinical advisory boards of Hemoshear and Moderna. A. C. received financial support from Nutricia, Vitaflo and Recordati/Orphan Europe, including research funds, institutional honoraria and consulting fees. M. D. has received reimbursement to attend symposia and fees for speaking at an organised meeting from Vitaflo and Nutricia and consultancy fees from Nutricia, Vitaflo and Recordati Rare Diseases. The other authors do not declare any conflict of interest related to this work.
Figures

References
-
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐926. 10.1136/bmj.39489.470347.AD. - DOI - PMC - PubMed
-
- Shchelochkov OA, Carrillo N, Venditti C. GeneReviews®: propionic acidemia. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews((R)). Seattle, WA; University of Washington, National Center for Biotechnology Information; 1993.
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

