Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction
- PMID: 33595593
- PMCID: PMC7890451
- DOI: 10.1001/jamacardio.2020.7585
Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction
Abstract
Importance: Dapagliflozin has been shown to reduce the risk of cardiovascular death or worsening heart failure (HF) in patients with chronic HF and reduced ejection fraction (HFrEF). However, clinical inertia often underlies deferred initiation of effective therapies.
Objective: To examine timing of onset of clinical benefit with dapagliflozin and magnitude as a function of proximity to prior HF hospitalization.
Design, setting, and participants: This is a secondary analysis of a completed multinational trial. The Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure trial was a double-blind, placebo-controlled randomized clinical trial of dapagliflozin in patients with chronic HFrEF (n = 4744). From February 2017 to August 2018, the study enrolled patients in New York Heart Association classes II through IV and with left ventricular ejection fraction of 40% or less; the median (range) follow-up time was 18.2 (0-27.8) months. Hazard ratios (HRs) were calculated for the primary efficacy outcome with dapagliflozin vs placebo by time following randomization. Efficacy and safety of dapagliflozin were assessed according to the timing of the most recent HF hospitalization prior to trial enrollment.
Exposures: None.
Main outcomes and measures: Composite of cardiovascular death or worsening HF.
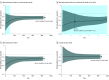
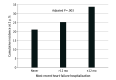
Results: A total of 4744 patients were included (1109 women [23.4%]; mean [SD] age, 66.3 [10.9] years). The reduction in the primary outcome with dapagliflozin was rapidly apparent, with a sustained statistically significant benefit by 28 days after randomization (HR at 28 days, 0.51 [95% CI, 0.28-0.94]; P = .03). A total of 2251 patients (47.4%) had been previously hospitalized for HF, and 1301 (27.4%) had been hospitalized within 12 months prior to enrollment. Among patients treated with placebo, there was a stepwise gradient of risk for the primary outcome according to timing of most recent HF hospitalization, with 2-year Kaplan-Meier rates of 21.1%, 25.3%, and 33.8% (adjusted P = .003) for patients with a prior HF hospitalization never, more than 12 months ago, and 12 or fewer months ago, respectively. Across these subgroups, dapagliflozin reduced the relative risk of the primary outcome by 16% (HR, 0.84 [95% CI, 0.69-1.01]), 27% (HR, 0.73 [95% CI, 0.54-0.99]), and 36% (HR, 0.64 [95% CI, 0.51-0.80]), respectively (P = .07 for trend). Accordingly, patients with a more recent HF hospitalization tended to experience greater absolute risk reductions with dapagliflozin at 2 years: 2.1% (95% CI, -1.9% to 6.1%), 4.1% (95% CI, -3.6% to 11.7%), and 9.9% (95% CI, 3.3%-16.5%), respectively (P = .05 for trend).
Conclusions and relevance: In this study, treatment with dapagliflozin was associated with rapid reduction in the risk of cardiovascular death or worsening HF, with a sustained statistically significant benefit emerging very early after randomization. Patients with a more recent HF hospitalization were at particularly high risk and experienced greater relative and absolute risk reductions with dapagliflozin.
Trial registration: ClinicalTrials.gov Identifier NCT03036124.
Conflict of interest statement
Figures

Comment in
-
Expediting the Benefits of Sodium-Glucose Cotransporter-2 Inhibitors for Heart Failure-There Is No Time for Delay.JAMA Cardiol. 2021 May 1;6(5):507-508. doi: 10.1001/jamacardio.2020.7596. JAMA Cardiol. 2021. PMID: 33595602 No abstract available.
References
-
- Solomon SD, Dobson J, Pocock S, et al. ; Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Investigators . Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482-1487. doi:10.1161/CIRCULATIONAHA.107.696906 - DOI - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, et al. . 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161. doi:10.1161/CIR.0000000000000509 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

