Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial
- PMID: 33595634
- PMCID: PMC7890452
- DOI: 10.1001/jama.2020.26848
Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial
Abstract
Importance: The efficacy of vitamin D3 supplementation in coronavirus disease 2019 (COVID-19) remains unclear.
Objective: To investigate the effect of a single high dose of vitamin D3 on hospital length of stay in patients with COVID-19.
Design, setting, and participants: This was a multicenter, double-blind, randomized, placebo-controlled trial conducted in 2 sites in Sao Paulo, Brazil. The study included 240 hospitalized patients with COVID-19 who were moderately to severely ill at the time of enrollment from June 2, 2020, to August 27, 2020. The final follow-up was on October 7, 2020.
Interventions: Patients were randomly assigned to receive a single oral dose of 200 000 IU of vitamin D3 (n = 120) or placebo (n = 120).
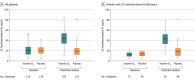
Main outcomes and measures: The primary outcome was length of stay, defined as the time from the date of randomization to hospital discharge. Prespecified secondary outcomes included mortality during hospitalization; the number of patients admitted to the intensive care unit; the number of patients who required mechanical ventilation and the duration of mechanical ventilation; and serum levels of 25-hydroxyvitamin D, total calcium, creatinine, and C-reactive protein.
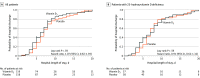
Results: Of 240 randomized patients, 237 were included in the primary analysis (mean [SD] age, 56.2 [14.4] years; 104 [43.9%] women; mean [SD] baseline 25-hydroxyvitamin D level, 20.9 [9.2] ng/mL). Median (interquartile range) length of stay was not significantly different between the vitamin D3 (7.0 [4.0-10.0] days) and placebo groups (7.0 [5.0-13.0] days) (log-rank P = .59; unadjusted hazard ratio for hospital discharge, 1.07 [95% CI, 0.82-1.39]; P = .62). The difference between the vitamin D3 group and the placebo group was not significant for in-hospital mortality (7.6% vs 5.1%; difference, 2.5% [95% CI, -4.1% to 9.2%]; P = .43), admission to the intensive care unit (16.0% vs 21.2%; difference, -5.2% [95% CI, -15.1% to 4.7%]; P = .30), or need for mechanical ventilation (7.6% vs 14.4%; difference, -6.8% [95% CI, -15.1% to 1.2%]; P = .09). Mean serum levels of 25-hydroxyvitamin D significantly increased after a single dose of vitamin D3 vs placebo (44.4 ng/mL vs 19.8 ng/mL; difference, 24.1 ng/mL [95% CI, 19.5-28.7]; P < .001). There were no adverse events, but an episode of vomiting was associated with the intervention.
Conclusions and relevance: Among hospitalized patients with COVID-19, a single high dose of vitamin D3, compared with placebo, did not significantly reduce hospital length of stay. The findings do not support the use of a high dose of vitamin D3 for treatment of moderate to severe COVID-19.
Trial registration: ClinicalTrials.gov Identifier: NCT04449718.
Conflict of interest statement
Figures

Comment in
-
Vitamin D3 to Treat COVID-19: Different Disease, Same Answer.JAMA. 2021 Mar 16;325(11):1047-1048. doi: 10.1001/jama.2020.26850. JAMA. 2021. PMID: 33595641 Free PMC article. No abstract available.
-
Learning from previous methodological pitfalls to propose well-designed trials on vitamin D in COVID-19.J Steroid Biochem Mol Biol. 2021 Jul;211:105901. doi: 10.1016/j.jsbmb.2021.105901. Epub 2021 Apr 14. J Steroid Biochem Mol Biol. 2021. PMID: 33864925 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

