Trends in Hepatocellular Carcinoma Incidence and Risk Among Persons With HIV in the US and Canada, 1996-2015
- PMID: 33595662
- PMCID: PMC7890526
- DOI: 10.1001/jamanetworkopen.2020.37512
Trends in Hepatocellular Carcinoma Incidence and Risk Among Persons With HIV in the US and Canada, 1996-2015
Abstract
Importance: People with HIV (PWH) are often coinfected with hepatitis B virus (HBV) and/or hepatitis C virus (HCV), leading to increased risk of developing hepatocellular carcinoma (HCC), but few cohort studies have had sufficient power to describe the trends of HCC incidence and risk among PWH in the combination antiretroviral therapy (cART) era.
Objective: To determine the temporal trends of HCC incidence rates (IRs) and to compare rates by risk factors among PWH in the cART era.
Design, setting, and participants: This cohort study used data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) study, which was conducted between 1996 and 2015. NA-ACCORD pooled individual-level data from 22 HIV clinical and interval cohorts of PWH in the US and Canada. PWH aged 18 years or older with available CD4 cell counts and HIV RNA data were enrolled. Data analyses were completed in March 2020.
Exposures: HBV infection was defined as detection of either HBV surface antigen, HBV e antigen, or HBV DNA in serum or plasma any time during observation. HCV infection was defined by detection of anti-HCV seropositivity, HCV RNA, or detectable genotype in serum or plasma at any time under observation.
Main outcomes and measures: HCC diagnoses were identified on the basis of review of medical records or cancer registry linkage.
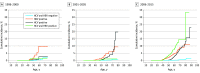
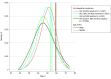
Results: Of 109 283 PWH with 723 441 person-years of follow-up, the median (interquartile range) age at baseline was 43 (36-51) years, 93 017 (85.1%) were male, 44 752 (40.9%) were White, 44 322 (40.6%) were Black, 21 343 (19.5%) had HCV coinfection, 6348 (5.8%) had HBV coinfection, and 2082 (1.9%) had triple infection; 451 individuals received a diagnosis of HCC by 2015. Between the early (1996-2000) and modern (2006-2015) cART eras, the crude HCC IR increased from 0.28 to 0.75 case per 1000 person-years. HCC IRs remained constant among HIV-monoinfected persons or those coinfected with HBV, but from 1996 to 2015, IRs increased among PWH coinfected with HCV (from 0.34 cases/1000 person-years in 1996 to 2.39 cases/1000 person-years in 2015) or those with triple infection (from 0.65 cases/1000 person-years in 1996 to 4.49 cases/1000 person-years in 2015). Recent HIV RNA levels greater than or equal to 500 copies/mL (IR ratio, 1.8; 95% CI, 1.4-2.4) and CD4 cell counts less than or equal to 500 cells/μL (IR ratio, 1.3; 95% CI, 1.0-1.6) were associated with higher HCC risk in the modern cART era. People who injected drugs had higher HCC risk compared with men who had sex with men (IR ratio, 2.0; 95% CI, 1.3-2.9), adjusted for HBV-HCV coinfection.
Conclusions and relevance: HCC rates among PWH increased significantly over time from 1996 to 2015. PWH coinfected with viral hepatitis, those with higher HIV RNA levels or lower CD4 cell counts, and those who inject drugs had higher HCC risk.
Conflict of interest statement
Figures
References
-
- Akinyemiju T, Abera S, Ahmed M, et al. ; Global Burden of Disease Liver Cancer Collaboration . The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683-1691. doi:10.1001/jamaoncol.2017.3055 - DOI - PMC - PubMed
-
- Silverberg MJ, Lau B, Achenbach CJ, et al. ; North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS . Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507-518. doi:10.7326/M14-2768 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 DA037788/DA/NIDA NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- U01 HL146241/HL/NHLBI NIH HHS/United States
- R01 AA016893/AA/NIAAA NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- KL2 TR000421/TR/NCATS NIH HHS/United States
- R01 DA012568/DA/NIDA NIH HHS/United States
- U01 AA020790/AA/NIAAA NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- P30 MH062246/MH/NIMH NIH HHS/United States
- U10 EY008057/EY/NEI NIH HHS/United States
- TGF-96118/CIHR/Canada
- U01 DA036935/DA/NIDA NIH HHS/United States
- R34 DA045592/DA/NIDA NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 HL146194/HL/NHLBI NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- N02CP55504/CP/NCI NIH HHS/United States
- R01 AG053100/AG/NIA NIH HHS/United States
- F31 AI124794/AI/NIAID NIH HHS/United States
- U01 HL146202/HL/NHLBI NIH HHS/United States
- U24 AA020794/AA/NIAAA NIH HHS/United States
- G12 MD007583/MD/NIMHD NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U10 EY008052/EY/NEI NIH HHS/United States
- U01 HL146245/HL/NHLBI NIH HHS/United States
- CBR-94036/CIHR/Canada
- U01 AA013566/AA/NIAAA NIH HHS/United States
- U01 HL146205/HL/NHLBI NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- K24 DA000432/DA/NIDA NIH HHS/United States
- U10 EY008067/EY/NEI NIH HHS/United States
- K01 AI093197/AI/NIAID NIH HHS/United States
- U54 MD007587/MD/NIMHD NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- U01 HL146333/HL/NHLBI NIH HHS/United States
- U01 HL146240/HL/NHLBI NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- R01 DA011602/DA/NIDA NIH HHS/United States
- K24 AI065298/AI/NIAID NIH HHS/United States
- U01 HL146242/HL/NHLBI NIH HHS/United States
- K24 AI118591/AI/NIAID NIH HHS/United States
- U01 HL146204/HL/NHLBI NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- HCP-97105/CIHR/Canada
- K01 AI131895/AI/NIAID NIH HHS/United States
- U01 HL146192/HL/NHLBI NIH HHS/United States
- UL1 TR000083/TR/NCATS NIH HHS/United States
- CBR-86906/CIHR/Canada
- U01 HL146208/HL/NHLBI NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- U01 HL146193/HL/NHLBI NIH HHS/United States
- Z01 CP010176/ImNIH/Intramural NIH HHS/United States
- U01 HL146201/HL/NHLBI NIH HHS/United States
- U01 AI069918/AI/NIAID NIH HHS/United States
- U01 HL146203/HL/NHLBI NIH HHS/United States
- K23 EY013707/EY/NEI NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

