Trilaciclib dose selection: an integrated pharmacokinetic and pharmacodynamic analysis of preclinical data and Phase Ib/IIa studies in patients with extensive-stage small cell lung cancer
- PMID: 33595690
- PMCID: PMC8026479
- DOI: 10.1007/s00280-021-04239-9
Trilaciclib dose selection: an integrated pharmacokinetic and pharmacodynamic analysis of preclinical data and Phase Ib/IIa studies in patients with extensive-stage small cell lung cancer
Abstract
Purpose: Trilaciclib is a first-in-class CDK4/6 inhibitor that transiently arrests hematopoietic stem and progenitor cells (HSPCs) in the G1 phase of the cell cycle to preserve them from chemotherapy-induced damage (myelopreservation). We report integrated analyses of preclinical and clinical data that informed selection of the recommended Phase II dose (RP2D) used in trilaciclib trials in extensive-stage small cell lung cancer (ES-SCLC).
Methods: A semi-mechanistic pharmacokinetic/pharmacodynamic (PK/PD) model developed from preclinical data guided selection of an optimal dose for G1 bone marrow arrest in a first-in-human Phase I study (G1T28-1-01). PK, PD, safety, and efficacy data from G1T28-1-01 and two Phase Ib/IIa studies (G1T28-02/-03) in ES-SCLC were analyzed to support RP2D selection.
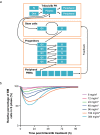
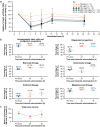
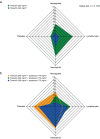
Results: Model simulation of bone marrow arrest based on preclinical data predicted that a ≥ 192 mg/m2 dose would induce a 40-50% decrease in total bone marrow proliferation in humans and almost 100% cell cycle arrest of cycling HSPCs. Consistent with this model, analysis of bone marrow aspirates in healthy volunteers after trilaciclib 192 mg/m2 administration demonstrated almost 100% G1 arrest in HSPCs and 40% decrease in total bone marrow proliferation, with minimal toxicity. G1T28-02/-03 reported similar PK parameters with trilaciclib 200 mg/m2 but slightly lower exposures than expected compared with healthy volunteers; consequently, 240 and 280 mg/m2 doses were also tested to match healthy volunteer exposures. Based on PK and relevant safety data, 240 mg/m2 was selected as the RP2D, which was also favored by myelopreservation endpoints in G1T28-02/-03.
Conclusion: Integrated PK/PD, safety, and efficacy data support 240 mg/m2 as the RP2D for trilaciclib. CLINICALTRIALS.
Gov identifiers: NCT02243150; NCT02499770; NCT02514447.
Keywords: CDK4/6 inhibitor; Chemotherapy-induced myelosuppression; Myelopreservation; Pharmacodynamics; Pharmacokinetics; Trilaciclib.
Conflict of interest statement
Chao Li: employee of G1 Therapeutics, Inc. at time of study. Lowell Hart: consultancy fees and research funding to institution from G1 Therapeutics, Inc. Taofeek K. Owonikoko: none to declare regarding this work. Raid Aljumaily: none to declare regarding this work. Caio Max Rocha Lima: none to declare regarding this work. Paul R. Conkling: research funding from Ignyta, Roche, BMS, Arcus Bioscience, Janssen, Aptose, and US Oncology Research. Roy Timothy Webb: none to declare regarding this work. Robert M. Jotte: none to declare regarding this work. Steven Schuster: none to declare regarding this work. William J. Edenfield: consultancy fees from Chimerix. Deborah A. Smith: consultancy fees from G1 Therapeutics, Inc. Mark Sale: consultancy fees from G1 Therapeutics, Inc. Patrick J. Roberts: employee of G1 Therapeutics, Inc. at time of study. Rajesh K. Malik: employee of G1 Therapeutics, Inc. Jessica A. Sorrentino: employee of G1 Therapeutics, Inc.
Figures
References
-
- Lyman GH. Chemotherapy dose intensity and quality cancer care. Oncol (Williston Park) 2006;20(14 Suppl 9):16–25. - PubMed
-
- Weiss JM, Csoszi T, Maglakelidze M, Hoyer RJ, Beck JT, Domine Gomez M, Lowczak A, Aljumaily R, Rocha Lima CM, Boccia RV, Hanna W, Nikolinakos P, Chiu VK, Owonikoko TK, Schuster SR, Hussein MA, Richards DA, Sawrycki P, Bulat I, Hamm JT, Hart LL, Adler S, Antal JM, Lai AY, Sorrentino JA, Yang Z, Malik RK, Morris SR, Roberts PJ, Dragnev KH. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol. 2019;30(10):1613–1621. doi: 10.1093/annonc/mdz278. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

