Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage
- PMID: 33595762
- PMCID: PMC8338793
- DOI: 10.1007/s10974-021-09596-9
Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage
Abstract
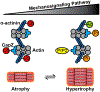
A transduced mechanical signal arriving at its destination in muscle alters sarcomeric structure and function. A major question addressed is how muscle mass and tension generation are optimized to match actual performance demands so that little energy is wasted. Three cases for improved energy efficiency are examined: the troponin complex for tuning force production, control of the myosin heads in a resting state, and the Z-disc proteins for sarcomere assembly. On arrival, the regulation of protein complexes is often controlled by post-translational modification (PTM), of which the most common are phosphorylation by kinases, deacetylation by histone deacetylases and ubiquitination by E3 ligases. Another branch of signals acts not through peptide covalent bonding but via ligand interactions (e.g. Ca2+ and phosphoinositide binding). The myosin head and the regulation of its binding to actin by the troponin complex is the best and earliest example of signal destinations that modify myofibrillar contractility. PTMs in the troponin complex regulate both the efficiency of the contractile function to match physiologic demand for work, and muscle mass via protein degradation. The regulation of sarcomere assembly by integration of incoming signaling pathways causing the same PTMs or ligand binding are discussed in response to mechanical loading and unloading by the Z-disc proteins CapZ, α-actinin, telethonin, titin N-termini, and others. Many human mutations that lead to cardiomyopathy and heart disease occur in the proteins discussed above, which often occur at their PTM or ligand binding sites.
Keywords: Mechanotransduction; Myofibrillogenesis; Phosphatidylinositol 4,5-bisphosphate; Proteomics; Signaling pathway.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG part of Springer Nature.
Conflict of interest statement
Compliance with ethical standards
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Myofibril growth during cardiac hypertrophy is regulated through dual phosphorylation and acetylation of the actin capping protein CapZ.Cell Signal. 2016 Aug;28(8):1015-24. doi: 10.1016/j.cellsig.2016.05.011. Epub 2016 May 13. Cell Signal. 2016. PMID: 27185186 Free PMC article.
-
Cyclic mechanical strain of myocytes modifies CapZβ1 post translationally via PKCε.J Muscle Res Cell Motil. 2015 Oct;36(4-5):329-37. doi: 10.1007/s10974-015-9420-6. Epub 2015 Oct 1. J Muscle Res Cell Motil. 2015. PMID: 26429793 Free PMC article.
-
Localization of CapZ during myofibrillogenesis in cultured chicken muscle.Cell Motil Cytoskeleton. 1993;25(4):317-35. doi: 10.1002/cm.970250403. Cell Motil Cytoskeleton. 1993. PMID: 8402953
-
Striated muscle proteins are regulated both by mechanical deformation and by chemical post-translational modification.Biophys Rev. 2021 Sep 4;13(5):679-695. doi: 10.1007/s12551-021-00835-4. eCollection 2021 Oct. Biophys Rev. 2021. PMID: 34777614 Free PMC article. Review.
-
Titin-based tension in the cardiac sarcomere: molecular origin and physiological adaptations.Prog Biophys Mol Biol. 2012 Oct-Nov;110(2-3):204-17. doi: 10.1016/j.pbiomolbio.2012.08.003. Epub 2012 Aug 11. Prog Biophys Mol Biol. 2012. PMID: 22910434 Free PMC article. Review.
Cited by
-
Alterations of Lysine Acetylation Profile in Murine Skeletal Muscles Upon Exercise.Front Aging Neurosci. 2022 May 3;14:859313. doi: 10.3389/fnagi.2022.859313. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35592697 Free PMC article.
-
The role of mechanosignaling in the control of myocardial mass.Am J Physiol Heart Circ Physiol. 2025 Mar 1;328(3):H622-H638. doi: 10.1152/ajpheart.00277.2024. Epub 2024 Dec 31. Am J Physiol Heart Circ Physiol. 2025. PMID: 39739566 Free PMC article. Review.
-
Myofilament-associated proteins with intrinsic disorder (MAPIDs) and their resolution by computational modeling.Q Rev Biophys. 2023 Jan 11;56:e2. doi: 10.1017/S003358352300001X. Q Rev Biophys. 2023. PMID: 36628457 Free PMC article. Review.
-
Cardiac Sarcomere Signaling in Health and Disease.Int J Mol Sci. 2022 Dec 19;23(24):16223. doi: 10.3390/ijms232416223. Int J Mol Sci. 2022. PMID: 36555864 Free PMC article. Review.
-
Cardiomyocyte external mechanical unloading activates modifications of α-actinin differently from sarcomere-originated unloading.FEBS J. 2023 Nov;290(22):5322-5339. doi: 10.1111/febs.16925. Epub 2023 Aug 17. FEBS J. 2023. PMID: 37551968 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

