Biologics and Targeted Synthetic Drugs Can Induce Immune-Mediated Glomerular Disorders in Patients with Rheumatic Diseases: An Updated Systematic Literature Review
- PMID: 33595833
- PMCID: PMC7952370
- DOI: 10.1007/s40259-021-00467-w
Biologics and Targeted Synthetic Drugs Can Induce Immune-Mediated Glomerular Disorders in Patients with Rheumatic Diseases: An Updated Systematic Literature Review
Abstract
Objective: Our objective was to update the understanding of the development of paradoxical immune-mediated glomerular disorders (IGDs) in patients with rheumatic diseases treated with biologics and targeted synthetic drugs (ts-drugs).
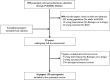
Methods: A systematic literature review was performed by searching PubMed for articles published between 1 January 2014 and 1 January 2020 reporting on the development of IGD in adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis or systemic lupus erythematosus (SLE) who were receiving biologics or ts-drugs. IGDs were classified on the basis of clinical, laboratory and histopathological data as (1) glomerulonephritis associated with systemic vasculitis (GNSV), (2) isolated autoimmune renal disorder (IARD) or (3) glomerulonephritis in SLE and in lupus-like syndrome (GNLS). The World Health Organization-Uppsala Monitoring Centre (WHO-UMC) system for standardized case causality assessment was applied to evaluate the causal relationship between IGD and specific drugs. The classification was based on a six-category scale, where the "certain" and "probable" categories were deemed clinically relevant relationships.
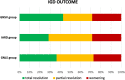
Results: The literature search retrieved 875 articles. Of these, 16 articles reported IGD data, for a total of 25 cases. According to the WHO-UMC assessment, the strength of the causal relationship between IGDs and investigated drugs was higher for anti-tumor necrosis factor-α agents (a clinically relevant relationship was found in four of six cases), abatacept (one of two cases), tocilizumab (two cases), ustekinumab (one case) and tofacitinib (one case) than for rituximab (nine cases), belimumab (three cases) or secukinumab (one case), which showed a weak causal relationship with these paradoxical events. No cases associated with apremilast or baricitinib were found. The retrieved cases were classified as 11 GNLS, seven IARD and seven GNSV.
Conclusions: Biologics and ts-drugs can cause IGDs. These events are rare, and the causative effect of a specific drug is hard to establish. When a patient is suspected of having an IGD, the drug should be discontinued, and treatment for the new-onset renal disorder should be promptly started.
Conflict of interest statement
AC has received consulting fees or honorarium for lectures or travel grants from Pfizer, BMS, Lilly, Janssen, Celgene, AbbVie, Novartis and MSD. EC, MP, AF, MC, IC and AM have no conflicts of interest that are directly relevant to the content of this article.
Figures
References
-
- Sepriano A, Kerschbaumer A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:760–770. doi: 10.1136/annrheumdis-2019-216653. - DOI - PubMed
-
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

