Application of the Milan System for Reporting Pediatric Salivary Gland Cytopathology: Analysis of histologic follow-up, risk of malignancy, and diagnostic accuracy
- PMID: 33595882
- PMCID: PMC10030063
- DOI: 10.1002/cncy.22415
Application of the Milan System for Reporting Pediatric Salivary Gland Cytopathology: Analysis of histologic follow-up, risk of malignancy, and diagnostic accuracy
Abstract
Background: The diagnosis and management of salivary gland tumors in pediatric patients can be challenging. The utility of fine-needle aspiration (FNA) cytopathology and the performance of the Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) in this age group have not been systematically assessed. The paucity of data has contributed to the controversial role of FNA cytopathology in the presurgical management of these patients.
Methods: The authors retrospectively analyzed 104 pediatric salivary gland FNAs (2000-2020). A correlation with the available histopathologic follow-up (n = 54) was performed. The distribution percentages, the risk of neoplasm (RON), and the risk of malignancy (ROM) were assessed for each category of the MSRSGC.
Results: The overall sensitivity, specificity, negative predictive value, and positive predictive value of pediatric salivary gland FNAs were 80%, 97%, and 92%, respectively. The RON values for the nondiagnostic, nonneoplastic, atypia of undetermined significance, benign neoplasm, salivary gland neoplasm of uncertain malignant potential, suspicious for malignancy, and malignant categories were 60%, 11%, 100%, 100%, 100%, 100%, and 100%, respectively, whereas the ROM values were 0%, 11%, 100%, 6%, 67%, 100%, and 100%, respectively. The percentage of nonneoplastic FNAs was greater in comparison with the adult population (52% vs 8%). All neoplasms in patients aged 0 to 10 years were malignant, whereas benign neoplasms occurred only in patients aged ≥11 years; this supported an inverse correlation between age and malignancy rate in salivary gland neoplasms.
Conclusions: FNA cytopathology demonstrates excellent diagnostic performance in differentiating malignant and benign pediatric salivary gland lesions. The MSRSGC is a valuable tool for standardization of the reporting and preoperative risk stratification of these lesions.
Keywords: Milan System for Reporting Salivary Gland Cytopathology (MSRSGC); fine-needle aspiration (FNA); pediatric; risk of malignancy (ROM); risk of neoplasm (RON); salivary gland.
© 2021 American Cancer Society.
Figures
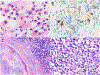

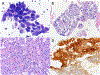
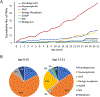
Similar articles
-
Application of the Milan System for Reporting Salivary Gland Cytopathology: A systematic review and meta-analysis.Cancer Cytopathol. 2022 Nov;130(11):849-859. doi: 10.1002/cncy.22604. Epub 2022 May 30. Cancer Cytopathol. 2022. PMID: 35637572 Free PMC article.
-
Application of the Milan System for Reporting Submandibular Gland Cytopathology: An international, multi-institutional study.Cancer Cytopathol. 2019 May;127(5):306-315. doi: 10.1002/cncy.22135. Epub 2019 May 3. Cancer Cytopathol. 2019. PMID: 31050186 Free PMC article.
-
Application of the Milan System for Reporting Salivary Gland Cytopathology in pediatric patients: An international, multi-institutional study.Cancer Cytopathol. 2022 May;130(5):370-380. doi: 10.1002/cncy.22556. Epub 2022 Jan 26. Cancer Cytopathol. 2022. PMID: 35081269
-
Comparative analysis of the World Health Organization Reporting System for Head and Neck Cytopathology and the Milan System for Reporting Salivary Gland Cytopathology.Cancer Cytopathol. 2025 Sep;133(9):e70041. doi: 10.1002/cncy.70041. Cancer Cytopathol. 2025. PMID: 40853710 Free PMC article.
-
Retrospective assessment of the effectiveness of the Milan system for reporting salivary gland cytology: A systematic review and meta-analysis of published literature.Diagn Cytopathol. 2019 Feb;47(2):67-87. doi: 10.1002/dc.24097. Epub 2018 Oct 29. Diagn Cytopathol. 2019. PMID: 30375201
Cited by
-
Epithelial salivary gland neoplasms in pediatric patients: A comprehensive review.Med Oral Patol Oral Cir Bucal. 2025 May 1;30(3):e440-e445. doi: 10.4317/medoral.26983. Med Oral Patol Oral Cir Bucal. 2025. PMID: 40192115 Free PMC article. Review.
-
Fine-Needle Aspiration Biopsy of Pediatric Salivary Gland Tumors: Analysis of Patient Tolerability, Sedation Requirement, and Procedural Complication.Acta Cytol. 2022;66(3):179-186. doi: 10.1159/000522208. Epub 2022 Feb 28. Acta Cytol. 2022. PMID: 35226899 Free PMC article.
-
Pediatric parotidectomy outcomes: A 14-year multicenter study.Laryngoscope Investig Otolaryngol. 2022 Nov 12;7(6):1875-1880. doi: 10.1002/lio2.975. eCollection 2022 Dec. Laryngoscope Investig Otolaryngol. 2022. PMID: 36544925 Free PMC article.
-
Application of the Milan System for Reporting Salivary Gland Cytopathology: A systematic review and meta-analysis.Cancer Cytopathol. 2022 Nov;130(11):849-859. doi: 10.1002/cncy.22604. Epub 2022 May 30. Cancer Cytopathol. 2022. PMID: 35637572 Free PMC article.
-
Nondiagnostic category of Milan System for Reporting Pediatric Salivary Gland Cytopathology: outcomes and root cause analysis.Cancer Cytopathol. 2022 Aug;130(8):609-619. doi: 10.1002/cncy.22571. Epub 2022 Mar 17. Cancer Cytopathol. 2022. PMID: 35298098 Free PMC article.
References
-
- Carlson ER, Schlieve T. Salivary Gland Malignancies. Oral Maxillofac Surg Clin North Am. 2019;31(1):125–144. - PubMed
-
- Lennon P, Silvera VM, Perez-Atayde A, Cunningham MJ, Rahbar R. Disorders and tumors of the salivary glands in children. Otolaryngol Clin North Am. 2015;48(1):153–173. - PubMed
-
- Luna MA, Batsakis JG, el-Naggar AK. Salivary gland tumors in children. Ann Otol Rhinol Laryngol. 1991;100(10):869–871. - PubMed
-
- Zamani M, Gronhoj C, Schmidt Jensen J, von Buchwald C, Charabi BW, Hjuler T. Survival and characteristics of pediatric salivary gland cancer: A systematic review and meta-analysis. Pediatr Blood Cancer. 2019;66(3):e27543. - PubMed
-
- Aro K, Leivo I, Makitie A. Management of salivary gland malignancies in the pediatric population. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):116–120. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

