Efficacy and safety of the fixed-ratio combination of insulin degludec and liraglutide by baseline glycated hemoglobin, body mass index and age in Japanese individuals with type 2 diabetes: A subgroup analysis of two phase III trials
- PMID: 33595901
- PMCID: PMC8409843
- DOI: 10.1111/jdi.13525
Efficacy and safety of the fixed-ratio combination of insulin degludec and liraglutide by baseline glycated hemoglobin, body mass index and age in Japanese individuals with type 2 diabetes: A subgroup analysis of two phase III trials
Abstract
Aims/introduction: To assess efficacy and safety of insulin degludec/liraglutide (IDegLira) in Japanese participants with type 2 diabetes across different baseline characteristics.
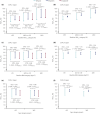
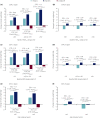
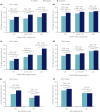
Materials and methods: Data from two randomized controlled trials were used: DUAL I Japan (n = 819 insulin-naïve participants) and DUAL II Japan (n = 210 insulin-experienced participants). Outcomes were assessed according to baseline glycated hemoglobin ( HbA1c ; <8.0%, ≥8.0-<9.0%, ≥9.0%), body mass index (<25, ≥25-<30, ≥30 kg/m2 ) and age (<65, ≥65 years).
Results: In DUAL I Japan, reductions in HbA1c with IDegLira versus degludec and liraglutide were observed across all subgroups (treatment differences: -0.48% to -0.72% vs degludec, -0.29% to -0.73% vs liraglutide). Results were similar with IDegLira versus degludec in DUAL II Japan (treatment differences: -0.82% to -1.61%). Treatment-by-subgroup interactions were significant for IDegLira versus liraglutide for baseline HbA1c and age in DUAL I Japan, and for IDegLira versus degludec for baseline HbA1c in DUAL II Japan. In DUAL I Japan, IDegLira was associated with less weight gain than degludec in most subgroups. In DUAL II Japan, IDegLira was associated with a small mean weight loss (except for baseline HbA1c ≥9.0%) versus a small gain for degludec (except for age ≥65 years subgroup); treatment-by-subgroup interactions were not significant. Total daily insulin dose was lower with IDegLira versus degludec across all categories, except for age >65 years in DUAL II Japan.
Conclusions: IDegLira reduced HbA1c in Japanese participants with type 2 diabetes across baseline HbA1c , body mass index and age categories, without unexpected safety issues.
Keywords: Insulin degludec/liraglutide; Japan; Type 2 diabetes mellitus.
© 2021 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
MK has received research support from Nippon Boehringer Ingelheim Co. Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd., MSD K.K., Takeda Pharmaceutical Company Ltd., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co. Ltd., Teijin Pharma Ltd., Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Daiichi Sankyo Company Ltd., Ono Pharmaceutical Co., Eli Lilly Japan K., Taisho Toyama Pharmaceutical Co., Ltd., Novartis Pharma K.K. and Kowa Pharmaceutical Co. Ltd; and has participated in speakers' bureaus for Nippon Boehringer Ingelheim Co. Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd., Msd K.K., Takeda Pharmaceutical Company Ltd., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Teijin Pharma Ltd., Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Terumo Corporation, Daiichi Sankyo Company Ltd., Eli Lilly Japan K., Taisho Toyama Pharmaceutical Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin Ltd., Medtronic Japan Co., Ltd. and Kowa Pharmaceutical Co. Ltd. HW has received grants from Kowa, Sanofi, Yakult, Eli Lilly, Novartis, Sanwa Kagaku Kenkyusho, Abbott Japan, Astellas Pharma, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Pfizer, Kissei Pharma, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme, Novo Nordisk, Ono Pharmaceutical, Teijin, Taisho‐Toyama and Souiken; and has received personal fees from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Dainippon Sumitomo Pharma, Eli Lilly, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, Kyowa Hakko Kirin, Terumo Corporation, Fuji Film and Takeda. SK has received honoraria for lectures from Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., AstraZeneca K.K. and Mitsubishi Tanabe Pharma Corporation. BRA and TN are employees and shareholders in Novo Nordisk. KK has received honoraria or consulting fees from Astellas Pharma, AstraZeneca, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, MSD, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Sanwa Kagaku Kenkyusho, Dainippon Sumitomo Pharma, Taisho Toyama Pharmaceutical and Takeda.
Figures
Similar articles
-
Superior HbA1c control with the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with a maximum dose of 50 units of insulin degludec in Japanese individuals with type 2 diabetes in a phase 3, double-blind, randomized trial.Diabetes Obes Metab. 2019 Dec;21(12):2694-2703. doi: 10.1111/dom.13859. Epub 2019 Sep 17. Diabetes Obes Metab. 2019. PMID: 31423685 Free PMC article. Clinical Trial.
-
Superior efficacy with a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with insulin degludec and liraglutide in insulin-naïve Japanese patients with type 2 diabetes in a phase 3, open-label, randomized trial.Diabetes Obes Metab. 2019 Dec;21(12):2674-2683. doi: 10.1111/dom.13856. Epub 2019 Aug 28. Diabetes Obes Metab. 2019. PMID: 31407845 Free PMC article. Clinical Trial.
-
Insulin degludec/liraglutide (IDegLira) was effective across a range of dysglycaemia and body mass index categories in the DUAL V randomized trial.Diabetes Obes Metab. 2018 Jan;20(1):200-205. doi: 10.1111/dom.13043. Epub 2017 Jul 31. Diabetes Obes Metab. 2018. PMID: 28643425 Free PMC article. Clinical Trial.
-
Efficacy and safety of fixed-ratio combination insulin degludec/liraglutide in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials.Diabetes Metab Res Rev. 2024 Mar;40(3):e3752. doi: 10.1002/dmrr.3752. Epub 2023 Nov 27. Diabetes Metab Res Rev. 2024. PMID: 38013215
-
Fixed-ratio combination therapy with GLP-1 receptor agonist liraglutide and insulin degludec in people with type 2 diabetes.Expert Rev Clin Pharmacol. 2017 Jun;10(6):621-632. doi: 10.1080/17512433.2017.1313109. Epub 2017 Apr 11. Expert Rev Clin Pharmacol. 2017. PMID: 28349716 Review.
Cited by
-
Retrospective Study of IDegLira, a New Fixed-Ratio Combination, in Japanese Patients With Type 2 Diabetes Mellitus: Analysis of Background Factors Affecting Effectiveness After 6 Months of Treatment.J Clin Med Res. 2023 Sep;15(8-9):406-414. doi: 10.14740/jocmr4995. Epub 2023 Sep 30. J Clin Med Res. 2023. PMID: 37822852 Free PMC article.
-
Switching from Insulin Degludec plus Dipeptidyl Peptidase-4 Inhibitor to Insulin Degludec/Liraglutide Improves Glycemic Variability in Patients with Type 2 Diabetes: A Preliminary Prospective Observation Study.J Diabetes Res. 2022 Jan 19;2022:5603864. doi: 10.1155/2022/5603864. eCollection 2022. J Diabetes Res. 2022. PMID: 35097130 Free PMC article.
-
Degree of obesity and gastrointestinal adverse reactions influence the weight loss effect of liraglutide in overweight or obese patients with type 2 diabetes.Ther Adv Chronic Dis. 2023 Mar 18;14:20406223231161516. doi: 10.1177/20406223231161516. eCollection 2023. Ther Adv Chronic Dis. 2023. PMID: 36950020 Free PMC article.
-
Effect of patient characteristics on the efficacy and safety of imeglimin monotherapy in Japanese patients with type 2 diabetes mellitus: A post-hoc analysis of two randomized, placebo-controlled trials.J Diabetes Investig. 2023 Sep;14(9):1101-1109. doi: 10.1111/jdi.14035. Epub 2023 Jun 1. J Diabetes Investig. 2023. PMID: 37264517 Free PMC article. Clinical Trial.
-
Efficacy and safety outcomes of dulaglutide by baseline HbA1c: A post hoc analysis of the REWIND trial.Diabetes Obes Metab. 2022 Sep;24(9):1753-1761. doi: 10.1111/dom.14760. Epub 2022 May 30. Diabetes Obes Metab. 2022. PMID: 35546279 Free PMC article.
References
-
- Kapitza C, Bode B, Ingwersen SH, et al. Preserved pharmacokinetic exposure and distinct glycemic effects of insulin degludec and liraglutide in IDegLira, a fixed‐ratio combination therapy. J Clin Pharmacol 2015; 55: 1369–1377. - PubMed
-
- Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014; 37: 2926–2933. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

