Temporal manipulation of KCC3 expression in juvenile or adult mice suggests irreversible developmental deficit in hereditary motor sensory neuropathy with agenesis of the corpus callosum
- PMID: 33596149
- PMCID: PMC8163575
- DOI: 10.1152/ajpcell.00594.2020
Temporal manipulation of KCC3 expression in juvenile or adult mice suggests irreversible developmental deficit in hereditary motor sensory neuropathy with agenesis of the corpus callosum
Abstract
Hereditary motor sensory neuropathy (HMSN/ACC) with agenesis of the corpus callosum (ACC) has been documented in the French-derived populations of Charlevoix and Saguenay/Lac St. Jean in Quebec, Canada, as well as a few sporadic families throughout the world. HMSN/ACC occurs because of loss-of-function mutations in the potassium-chloride cotransporter 3 (KCC3). In HMSN/ACC, motor deficits occur early in infancy with rapid and continual deterioration of motor and sensory fibers into juvenile and adulthood. Genetic work in mice has demonstrated that the disease is caused by loss of KCC3 function in neurons and particularly parvalbumin (PV)-expressing neurons. Currently, there are no treatments or cures for HMSN/ACC other than pain management. As genetic counseling in Quebec has increased as a preventative strategy, most individuals with HSMN/ACC are now adults. The onset of the disease is unknown. In particular, it is unknown if the disease starts early during development and whether it can be reversed by restoring KCC3 function. In this study, we used two separate mouse models that when combined to the PV-CreERT2 tamoxifen-inducible system allowed us to 1) disrupt KCC3 expression in adulthood or juvenile periods; and 2) reintroduce KCC3 expression in mice that first develop with a nonfunctional cotransporter. We show that disrupting or reintroducing KCC3 in the adult mouse has no effect on locomotor behavior, indicating that expression of KCC3 is critical during embryonic development and/or the perinatal period and that once the disease has started, reexpressing a functional cotransporter fails to change the course of HMSN/ACC.
Keywords: Andermann Syndrome; HSMN/ACC; KCC3 mouse model; parvalbumin-CreERT2; tamoxifen.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




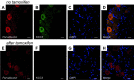

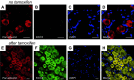
References
-
- Casaubon LK, Melanson M, Lopes-Cendes I, Marineau C, Andermann E, Andermann F, Weissenbach J, Prevost C, Bouchard JP, Mathieu J, Rouleau GA. The gene responsible for a severe form of peripheral neuropathy and agenesis of the corpus callosum maps to chromosome 15q. Am J Hum Genet 58: 28–34, 1996. - PMC - PubMed
-
- Howard HC, Mount DB, Rochefort D, Byun N, Dupré N, Lu J, Fan X, Song L, Rivière J-B, Prévost C, Horst J, Simonati A, Lemcke B, Welch R, England R, Zhan FQ, Mercado A, Siesser WB, George AL, McDonald MP, Bouchard JP, Mathieu J, Delpire E, Rouleau GA. Mutations in the K-Cl cotransporter KCC3 cause a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet 32: 384–392, 2002. [Erratum in Nat Genet 32: 681, 2002]. doi: 10.1038/ng1002. - DOI - PubMed
-
- Salin-Cantegrel A, Rivière JB, Dupré N, Charron FM, Shekarabi M, Karéméra L, Gaspar C, Horst J, Tekin M, Deda G, Krause A, Lippert MM, Willemsen MA, Jarrar R, Lapointe JY, Rouleau GA. Distal truncation of KCC3 in non-French Canadian HMSN/ACC families. Neurology 69: 1350–1355, 2007. doi: 10.1212/01.wnl.0000291779.35643.15. - DOI - PubMed
-
- Andermann F, Andermann E, Joubert M, Karpati G, Carpenter S, Melancon D. Familial agenesis of the corpus callosum with anterior horn cell disease: a syndrome of mental retardation, areflexia and paraparesis. Trans Am Neurol Assoc 97: 242–244, 1972.
-
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Leuwer R, Pape HC, Volkl H, Hubner CA, Jentsch TJ. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. EMBO J 22: 5422–5434, 2003. doi: 10.1093/emboj/cdg519. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 DK110375/DK/NIDDK NIH HHS/United States
- R03NS063090/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- RO1DK93501/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- P50 HD103537/HD/NICHD NIH HHS/United States
- R01 DK093501/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

