COVID-19 induces a hyperactive phenotype in circulating platelets
- PMID: 33596198
- PMCID: PMC7920383
- DOI: 10.1371/journal.pbio.3001109
COVID-19 induces a hyperactive phenotype in circulating platelets
Abstract
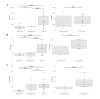
Coronavirus Disease 2019 (COVID-19), caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has affected over 30 million globally to date. Although high rates of venous thromboembolism and evidence of COVID-19-induced endothelial dysfunction have been reported, the precise aetiology of the increased thrombotic risk associated with COVID-19 infection remains to be fully elucidated. Therefore, we assessed clinical platelet parameters and circulating platelet activity in patients with severe and nonsevere COVID-19. An assessment of clinical blood parameters in patients with severe COVID-19 disease (requiring intensive care), patients with nonsevere disease (not requiring intensive care), general medical in-patients without COVID-19, and healthy donors was undertaken. Platelet function and activity were also assessed by secretion and specific marker analysis. We demonstrated that routine clinical blood parameters including increased mean platelet volume (MPV) and decreased platelet:neutrophil ratio are associated with disease severity in COVID-19 upon hospitalisation and intensive care unit (ICU) admission. Strikingly, agonist-induced ADP release was 30- to 90-fold higher in COVID-19 patients compared with hospitalised controls and circulating levels of platelet factor 4 (PF4), soluble P-selectin (sP-selectin), and thrombopoietin (TPO) were also significantly elevated in COVID-19. This study shows that distinct differences exist in routine full blood count and other clinical laboratory parameters between patients with severe and nonsevere COVID-19. Moreover, we have determined all COVID-19 patients possess hyperactive circulating platelets. These data suggest abnormal platelet reactivity may contribute to hypercoagulability in COVID-19 and confirms the role that platelets/clotting has in determining the severity of the disease and the complexity of the recovery path.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

