High throughput detection and genetic epidemiology of SARS-CoV-2 using COVIDSeq next-generation sequencing
- PMID: 33596239
- PMCID: PMC7888613
- DOI: 10.1371/journal.pone.0247115
High throughput detection and genetic epidemiology of SARS-CoV-2 using COVIDSeq next-generation sequencing
Abstract
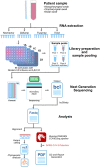
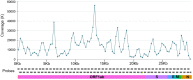
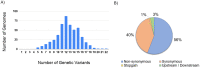
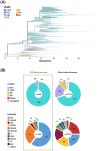
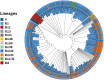
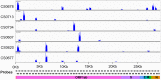
The rapid emergence of coronavirus disease 2019 (COVID-19) as a global pandemic affecting millions of individuals globally has necessitated sensitive and high-throughput approaches for the diagnosis, surveillance, and determining the genetic epidemiology of SARS-CoV-2. In the present study, we used the COVIDSeq protocol, which involves multiplex-PCR, barcoding, and sequencing of samples for high-throughput detection and deciphering the genetic epidemiology of SARS-CoV-2. We used the approach on 752 clinical samples in duplicates, amounting to a total of 1536 samples which could be sequenced on a single S4 sequencing flow cell on NovaSeq 6000. Our analysis suggests a high concordance between technical duplicates and a high concordance of detection of SARS-CoV-2 between the COVIDSeq as well as RT-PCR approaches. An in-depth analysis revealed a total of six samples in which COVIDSeq detected SARS-CoV-2 in high confidence which were negative in RT-PCR. Additionally, the assay could detect SARS-CoV-2 in 21 samples and 16 samples which were classified inconclusive and pan-sarbeco positive respectively suggesting that COVIDSeq could be used as a confirmatory test. The sequencing approach also enabled insights into the evolution and genetic epidemiology of the SARS-CoV-2 samples. The samples were classified into a total of 3 clades. This study reports two lineages B.1.112 and B.1.99 for the first time in India. This study also revealed 1,143 unique single nucleotide variants and added a total of 73 novel variants identified for the first time. To the best of our knowledge, this is the first report of the COVIDSeq approach for detection and genetic epidemiology of SARS-CoV-2. Our analysis suggests that COVIDSeq could be a potential high sensitivity assay for the detection of SARS-CoV-2, with an additional advantage of enabling the genetic epidemiology of SARS-CoV-2.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- de Lusignan S, Lopez Bernal J, Zambon M, Akinyemi O, Amirthalingam G, Andrews N, et al. Emergence of a Novel Coronavirus (COVID-19): Protocol for Extending Surveillance Used by the Royal College of General Practitioners Research and Surveillance Centre and Public Health England. JMIR Public Health Surveill. 2020;6: e18606 10.2196/18606 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

