Chiral Dibenzopentalene-Based Conjugated Nanohoops through Stereoselective Synthesis
- PMID: 33596338
- PMCID: PMC8252646
- DOI: 10.1002/anie.202016968
Chiral Dibenzopentalene-Based Conjugated Nanohoops through Stereoselective Synthesis
Abstract
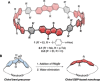
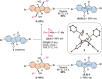
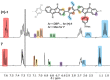
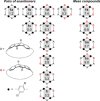
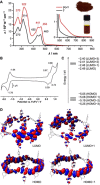
Conjugated nanohoops allow to investigate the effect of radial conjugation and bending on the involved π-systems. They can possess unexpected optoelectronic properties and their radially oriented π-system makes them attractive for host-guest chemistry. Bending the π-subsystems can lead to chiral hoops. Herein, we report the stereoselective synthesis of two enantiomers of chiral conjugated nanohoops by incorporating dibenzo[a,e]pentalenes (DBPs), which are generated in the last synthetic step from enantiomerically pure diketone precursors. Owing to its bent shape, this diketone unit was used as the only bent precursor and novel "corner unit" in the synthesis of the hoops. The [6]DBP[4]Ph-hoops contain six antiaromatic DBP units and four bridging phenylene groups. The small HOMO-LUMO gap and ambipolar electrochemical character of the DBP units is reflected in the optoelectronic properties of the hoop. Electronic circular dichroism spectra and MD simulations showed that the chiral hoop did not racemize even when heated to 110 °C. Due to its large diameter, it was able to accommodate two C60 molecules, as binding studies indicate.
Keywords: antiaromaticity; chiral macrocycles; chiral resolution; cycloparaphenylenes; fullerenes.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gleiter R., Haberhauer G., Aromaticity and Other Conjugation Effects, Wiley-VCH, Weinheim, 2012.
-
- Schröder A., Mekelburger H.-B., Vögtle F., in Cyclophanes (Ed.: Weber E.), Springer-Verlag, Berlin, Heidelberg, 1994, pp. 179–201.
-
- Tahara K., Tobe Y., Chem. Rev. 2006, 106, 5274–5290. - PubMed
-
- Takaba H., Omachi H., Yamamoto Y., Bouffard J., Itami K., Angew. Chem. Int. Ed. 2009, 48, 6112–6116; - PubMed
- Angew. Chem. 2009, 121, 6228–6232.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

