An mTORC1-dependent switch orchestrates the transition between mouse spermatogonial stem cells and clones of progenitor spermatogonia
- PMID: 33596419
- PMCID: PMC7980622
- DOI: 10.1016/j.celrep.2021.108752
An mTORC1-dependent switch orchestrates the transition between mouse spermatogonial stem cells and clones of progenitor spermatogonia
Abstract
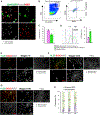
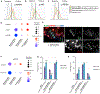
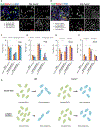
Spermatogonial stem cells (SSCs) sustain spermatogenesis by balancing self-renewal and initiation of differentiation to produce progenitor spermatogonia committed to forming sperm. To define the regulatory logic among SSCs and progenitors, we performed single-cell RNA velocity analyses and validated results in vivo. A predominant quiescent SSC population spawns a small subset of cell-cycle-activated SSCs via mitogen-activated protein kinase (MAPK)/AKT signaling. Activated SSCs form early progenitors and mTORC1 inhibition drives activated SSC accumulation consistent with blockade to progenitor formation. Mechanistically, mTORC1 inhibition suppresses transcription among spermatogonia and specifically alters expression of insulin growth factor (IGF) signaling in early progenitors. Tex14-/- testes lacking intercellular bridges do not accumulate activated SSCs following mTORC1 inhibition, indicating that steady-state mTORC1 signaling drives activated SSCs to produce progenitor clones. These results are consistent with a model of SSC self-renewal dependent on interconversion between activated and quiescent SSCs, and mTORC1-dependent initiation of differentiation from SSCs to progenitor clones.
Keywords: RNA velocity; cell fate; single cell; spermatogenesis; subpopulations; testis; undifferentiated spermatogonia.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

