Histatin 5 variant reduces Candida albicans biofilm viability and inhibits biofilm formation
- PMID: 33596477
- PMCID: PMC7940551
- DOI: 10.1016/j.fgb.2021.103529
Histatin 5 variant reduces Candida albicans biofilm viability and inhibits biofilm formation
Abstract
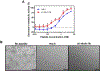
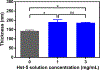
Candida albicans is a commensal organism and opportunistic pathogen that can form biofilms that colonize surfaces of medical devices, such as implants, catheters, and dentures. Compared to planktonic C. albicans cells, cells in biofilms exhibit increased resistance to treatment. Histatin 5 (Hst-5) is an antimicrobial peptide that is natively secreted by human salivary glands and has strong antifungal activity against C. albicans. However, C. albicans produces secreted aspartic proteases (Saps) that can cleave and inactivate Hst-5, limiting its antifungal properties. We previously showed that residue substitutions K11R and K17R within Hst-5 improve its antifungal activity and prevent proteolytic degradation by Saps when treating planktonic C. albicans. Here, we investigated the use of the K11R-K17R peptide as an alternative therapeutic against C. albicans biofilms by assessing its ability to reduce viability of pre-formed biofilms and to inhibit the formation of biofilms and showed that K11R-K17R had improved activity compared to Hst-5. Based on these results, we incorporated K11R-K17R and Hst-5 into polyelectrolyte multilayer (PEM) surface coatings and demonstrated that films functionalized with K11R-K17R reduced the formation of C. albicans biofilms. Our results demonstrate the therapeutic potential of the K11R-K17R Hst-5 variant in preventing and treating biofilms.
Keywords: Antimicrobial peptides; Biofilm; Candida albicans; Histatin 5; Polyelectrolyte multilayer film.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest
AJK is an inventor on a patent that includes the K11R-K17R peptide. CMJ is an employee of the VA Maryland Health Care System. The views reported in this paper do not reflect the views of the Department of Veterans Affairs or the United States Government. CMJ has an equity position in Cellth Systems, LLC, and Avidea Technologies.
Figures
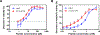


References
-
- Boudou T, et al. , 2010. Multiple functionalities of polyelectrolyte multilayer films: new biomedical applications. Adv Mater. 22, 441–67. - PubMed
-
- Centers for Disease Control and Prevention, Antibiotic Resistance Threats in the United States, 2019. Vol. 2020, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

