Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients
- PMID: 33596494
- PMCID: PMC7857981
- DOI: 10.1016/j.phymed.2021.153494
Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients
Abstract
Background: Specific treatment for COVID-19 is still an unmet need. Outcomes of clinical trials on repurposed drugs have not been yielding success. Therefore, it is necessary to include complementary approaches of medicine against COVID-19.
Purpose: This study was designed to evaluate the impact of traditional Indian Ayurvedic treatment regime on asymptomatic patients with COVID-19 infection.
Study design: It is a placebo controlled randomized double-blind pilot clinical trial.
Methods: The study was registered with Clinical Trial Registry-India (vide Registration No. CTRI/2020/05/025273) and conducted at the Department of Medicine in National Institute of Medical Sciences and Research, Jaipur, India. 1 g of Giloy Ghanvati (Tinospora cordifolia) and 2 g of Swasari Ras (traditional herbo-mineral formulation) and 0.5 g each of Ashwagandha (Withania somnifera) and Tulsi Ghanvati (Ocimum sanctum) were given orally to the patients in treatment group twice per day for 7 days. Medicines were given in the form of tablets and each tablet weighed 500 mg. While, Swasari Ras was administered in powdered form, 30 min before breakfasts and dinners, rest were scheduled for 30 min post-meals. Patients in the treatment group also received 4 drops of Anu taila (traditional nasal drop) in each nostril every day 1 h before breakfast. Patients in the placebo group received identical-looking tablets and drops, post randomization and double blinded assortments. RT-qPCR test was used for the detection of viral load in the nasopharyngeal and oropharyngeal swab samples of study participants during the study. Chemiluminescent immunometric assay was used to quantify serum levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and high sensitivity C-reactive protein (hs-CRP) on day 1 and day 7 of the study.
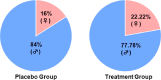
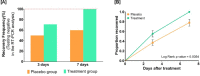
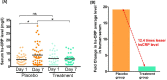
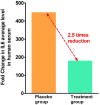
Results: By day 3, 71.1 % and 50.0 % patients recovered in the treatment and placebo groups, respectively. Treatment group witnessed 100 % recovery by day 7, while it was 60.0 % in the placebo group. Average fold changes in serum levels of hs-CRP, IL-6 and TNF-α in treatment group were respectively, 12.4, 2.5 and 20 times lesser than those in the placebo group at day 7. There was 40 % absolute reduction in the risk of delayed recovery from infection in the treatment group.
Conclusions: Ayurvedic treatment can expedite virological clearance, help in faster recovery and concomitantly reduce the risk of viral dissemination. Reduced inflammation markers suggested less severity of SARS-CoV-2 infection in the treatment group. Moreover, there was no adverse effect observed to be associated with this treatment.
Keywords: Anu taila; Ayurveda; COVID-19; Ocimum sanctum; Randomized placebo-controlled pilot clinical trial; Swasari Ras; Tinospora cordifolia; Withania somnifera.
Copyright © 2021. Published by Elsevier GmbH.
Conflict of interest statement
Authors declare no conflict of interests with regards to the submitted work. The medications were provided by Divya Pharmacy, Haridwar, Uttarakhand, India. Acharya Balkrishna is an honorary trustee in Divya Yog Mandir Trust. Besides, providing the medications, Divya Pharmacy was not involved in any aspect of the clinical trial reported in this study. Clinical trial was conducted at National Institute of Medical Sciences, Jaipur, India.
Figures

References
-
- Balkrishna A., Solleti S.K., Singh H., Tomer M., Sharma N., Varshney A. Calcio-herbal formulation, divya-swasari-ras, alleviates chronic inflammation and suppresses airway remodelling in mouse model of allergic asthma by modulating pro-inflammatory cytokine response. Biomed. Pharmacother. 2020;126 doi: 10.1016/j.biopha.2020.110063. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

