New Methods and Strategies in the Synthesis of Terpenoid Natural Products
- PMID: 33596652
- PMCID: PMC10122273
- DOI: 10.1021/acs.accounts.0c00809
New Methods and Strategies in the Synthesis of Terpenoid Natural Products
Abstract
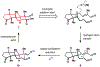
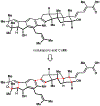
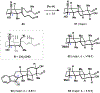
Indoloterpenoids of the paxilline type belong to a large family of secondary metabolites that exhibit unique molecular architectures and a diverse set of biological activities. More than 100 congeners identified to date share a common structural motif that contains an indole moiety fused to a rearranged diterpenoid fragment. The representative physiological and cellular effects attributed to this family of natural products include neurological and insecticidal activities, modulation of lipid balance, and inhibition of mitosis. The uniting polycyclic motif combined with the diversity of individual structural features of paxilline indoloterpenoids and the broad scope of their biological activities have fascinated organic chemists for the past four decades and have led to the development of numerous syntheses. In this Account, we describe our contributions to this field and how they in turn shape new directions that are developing in our laboratory.We begin with the discussion of our strategy for the synthesis of the shared indoloterpenoid core. To address stereochemical challenges encountered in earlier reports, we planned to leverage a suitably substituted cyclopentanone in a polycyclization to form the desired trans-decalin motif. This polycyclization relied on a radical-polar crossover cascade initiated by hydrogen atom transfer. The original process exhibited poor diastereoselectivity, but we discovered an efficient solution to this problem that took advantage of intramolecular tethering effects, culminating in short synthesis of emindole SB. During these studies, we also identified indium-mediated alkenylation of silyl enol ethers with alkynes as a suitable method for the synthesis of highly substituted β,γ-unsaturated ketones that was critical to achieving brevity of our route. We subsequently developed a catalytic version of this transformation that allowed for a formal bimolecular ene reaction that exhibited unusual and potentially useful selectivity in construction of quaternary centers.To test the scope and limitations of our approach to paxilline indoloterpenoids and identify potential improvements, we developed a synthesis of the more complex congener nodulisporic acid C. The convergent assembly of this natural product was enabled by identification of new elements of stereocontrol in the radical-polar crossover polycyclization en route to the polycyclic terpenoid motif and development of a highly diastereoselective enyne cycloisomerization to access the indenopyran motif and a ketone arylation protocol to unite the two complex fragments.In subsequent studies, we expanded the radical-polar crossover cascade underlying our approach to paxilline indoloterpenoids to a bimolecular setting, which allowed for annulation of two unsaturated carbonyl components to produce functionalized cyclohexanes. This transformation is particularly well suited for installation of fully substituted carbons and can be complementary to the venerable Diels-Alder reaction. The utility of the new annulation was tested in the synthesis of forskolin, allowing for rapid construction of the complex polycyclic motif in this densely functionalized labdane diterpenoid.Over the past five years, our initial forays into the synthesis of paxilline indoloterpenoids have grown into a program that incorporates development of new synthetic methods and pursues artificial assembly of terpenoid natural products from several different families. We are encouraged by the increasing diversity of structural motifs made accessible by application of this chemistry and continue to discover new aspects of the underlying reactivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
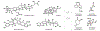
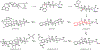
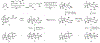
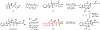
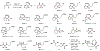
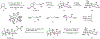
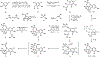

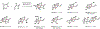

References
-
- George DT; Kuenstner EJ; Pronin SV A concise approach to paxilline indole diterpenes. J. Am. Chem. Soc 2015, 137, 15410. - PMC - PubMed
-
This report described our initial foray into the synthesis of paxilline indoloterpenoid, established the validity of our approach to the common polycyclic motif, and demonstrated synthesis of emindole SB as a proof-of-concept for application of our strategy.
-
- Holmbo SD; Godfrey NA; Hirner JJ; Pronin SV A catalytic intermolecular formal ene reaction between ketone-derived silyl enol ethers and alkynes. J. Am. Chem. Soc 2016, 138, 12316. - PMC - PubMed
-
This report described the development of a catalytic method for bimolecular alkenylation of ketone-derived silyl enol ethers with alkyne, a transformation that exhibits unusual selectivity in construction of quaternary centers and was key to success in our synthesis of emindole SB.
-
- Thomas WP; Schatz DJ; George DT; Pronin SV A Radical-Polar Crossover Annulation to Access Terpenoid Motifs. J. Am. Chem. Soc 2019, 141, 12246. - PMC - PubMed
-
This report detailed expansion of the radical-polar crossover cascade employed in our synthesis of paxilline indoloterpenoids to a bimolecular setting, allowing for annulation of two unsaturated carbonyl components that enabled a brief synthesis of forskolin.
-
-
For examples, see:
- Sheehan JC; Henery Logan KR A General Synthesis of the Penicillins. J. Am. Chem. Soc 1959, 81, 5838.
- Corey EJ; Weinshenker NM; Schaaf TK; Huber W Stereo-Controlled Synthesis of Prostaglandins F2α and E2 (dl). J. Am. Chem. Soc 1969, 91, 5675. - PubMed
- Aicher TD; Buszek KR; Fang FG; Forsyth CJ; Jung SH; Kishi Y; Matelich MC; Scola PM; Spero DM; Yoon SK Total Synthesis of Halichondrin B and Norhalichondrin B. J. Am. Chem. Soc 1992, 114, 3162.
- Corey EJ; Gin DY; Kania RS Enantioselective Total Synthesis of Ecteinascidin 743. J. Am. Chem. Soc 1996, 118, 9202.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

