The Whole Is Bigger than the Sum of Its Parts: Drug Transport in the Context of Two Membranes with Active Efflux
- PMID: 33596653
- PMCID: PMC8369882
- DOI: 10.1021/acs.chemrev.0c01137
The Whole Is Bigger than the Sum of Its Parts: Drug Transport in the Context of Two Membranes with Active Efflux
Abstract
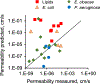
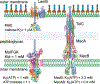
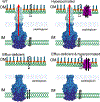
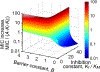
Cell envelope plays a dual role in the life of bacteria by simultaneously protecting it from a hostile environment and facilitating access to beneficial molecules. At the heart of this ability lie the restrictive properties of the cellular membrane augmented by efflux transporters, which preclude intracellular penetration of most molecules except with the help of specialized uptake mediators. Recently, kinetic properties of the cell envelope came into focus driven on one hand by the urgent need in new antibiotics and, on the other hand, by experimental and theoretical advances in studies of transmembrane transport. A notable result from these studies is the development of a kinetic formalism that integrates the Michaelis-Menten behavior of individual transporters with transmembrane diffusion and offers a quantitative basis for the analysis of intracellular penetration of bioactive compounds. This review surveys key experimental and computational approaches to the investigation of transport by individual translocators and in whole cells, summarizes key findings from these studies and outlines implications for antibiotic discovery. Special emphasis is placed on Gram-negative bacteria, whose envelope contains two separate membranes. This feature sets these organisms apart from Gram-positive bacteria and eukaryotic cells by providing them with full benefits of the synergy between slow transmembrane diffusion and active efflux.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

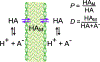




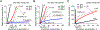
References
-
- Nikaido H Prevention of Drug Access to Bacterial Targets: Permeability Barriers and Active Efflux. Science 1994, 264, 382–388. - PubMed
-
- Silver LL A Gestalt Approach to Gram-Negative Entry. Bioorg. Med. Chem 2016, 24, 6379–6389. - PubMed
-
- Szakacs G; Paterson JK; Ludwig JA; Booth-Genthe C; Gottesman M. M Targeting Multidrug Resistance in Cancer. Nat. Rev. Drug Discovery 2006, 5, 219–234. - PubMed
-
- Antibiotic Resistance Threats in the United States, 2019; Center for Disease Control and Prevention, DHHS: Atlanta, GA, 2019; DOI: DOI: 10.15620/cdc:82532. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

