Ethnic variability associating gut and oral microbiome with obesity in children
- PMID: 33596768
- PMCID: PMC7894456
- DOI: 10.1080/19490976.2021.1882926
Ethnic variability associating gut and oral microbiome with obesity in children
Abstract
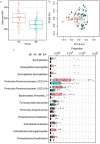
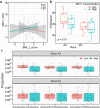
Obesity is a growing worldwide problem that generally starts in the early years of life and affects minorities more often than Whites. Thus, there is an urgency to determine factors that can be used as targets as indicators of obesity. In this study, we attempt to generate a profile of gut and oral microbial clades predictive of disease status in African American (AA) and European American (EA) children. 16S rDNA sequencing of the gut and saliva microbial profiles were correlated with salivary amylase, socioeconomic factors (e.g., education and family income), and obesity in both ethnic populations. Gut and oral microbial diversity between AA and EA children showed significant differences in alpha-, beta-, and taxa-level diversity. While gut microbial diversity between obese and non-obese was not evident in EA children, the abundance of gut Klebsiella and Magasphaera was associated with obesity in AA children. In contrast, an abundance of oral Aggregatibacter and Eikenella in obese EA children was observed. These observations suggest an ethnicity-specific association with gut and oral microbial profiles. Socioeconomic factors influenced microbiota in obesity, which were ethnicity dependent, suggesting that specific approaches to confront obesity are required for both populations.
Keywords: Microbiome; disparity; minorities; obesity; socioeconomic factors.
Figures




References
-
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–15. doi: 10.1016/S0140-6736(14)60460-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
