Integrated metabolic profiling and transcriptome analysis of pigment accumulation in Lonicera japonica flower petals during colour-transition
- PMID: 33596836
- PMCID: PMC7890969
- DOI: 10.1186/s12870-021-02877-y
Integrated metabolic profiling and transcriptome analysis of pigment accumulation in Lonicera japonica flower petals during colour-transition
Abstract
Background: Plants have remarkable diversity in petal colour through the biosynthesis and accumulation of various pigments. To better understand the mechanisms regulating petal pigmentation in Lonicera japonica, we used multiple approaches to investigate the changes in carotenoids, anthocyanins, endogenous hormones and gene expression dynamics during petal colour transitions, i.e., green bud petals (GB_Pe), white flower petals (WF_Pe) and yellow flower petals (YF_Pe).
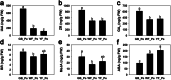
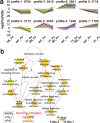
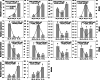
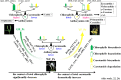
Results: Metabolome analysis showed that YF_Pe contained a much higher content of carotenoids than GB_Pe and WF_Pe, with α-carotene, zeaxanthin, violaxanthin and γ-carotene identified as the major carotenoid compounds in YF_Pe. Comparative transcriptome analysis revealed that the key differentially expressed genes (DEGs) involved in carotenoid biosynthesis, such as phytoene synthase, phytoene desaturase and ζ-carotene desaturase, were significantly upregulated in YF_Pe. The results indicated that upregulated carotenoid concentrations and carotenoid biosynthesis-related genes predominantly promote colour transition. Meanwhile, two anthocyanins (pelargonidin and cyanidin) were significantly increased in YF_Pe, and the expression level of an anthocyanidin synthase gene was significantly upregulated, suggesting that anthocyanins may contribute to vivid yellow colour in YF_Pe. Furthermore, analyses of changes in indoleacetic acid, zeatin riboside, gibberellic acid, brassinosteroid (BR), methyl jasmonate and abscisic acid (ABA) levels indicated that colour transitions are regulated by endogenous hormones. The DEGs involved in the auxin, cytokinin, gibberellin, BR, jasmonic acid and ABA signalling pathways were enriched and associated with petal colour transitions.
Conclusion: Our results provide global insight into the pigment accumulation and the regulatory mechanisms underlying petal colour transitions during the flower development process in L. japonica.
Keywords: Endogenous hormones; Gene expression; Lonicera japonica; Petal colour; Pigment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Carotenoid metabolomic and transcriptomic analyses provide insights into the flower color transition in Lonicera macranthoides.BMC Biotechnol. 2025 Jul 9;25(1):69. doi: 10.1186/s12896-025-01007-y. BMC Biotechnol. 2025. PMID: 40634871 Free PMC article.
-
Temporal and spatial regulation of anthocyanin biosynthesis provide diverse flower colour intensities and patterning in Cymbidium orchid.Planta. 2014 Nov;240(5):983-1002. doi: 10.1007/s00425-014-2152-9. Epub 2014 Sep 3. Planta. 2014. PMID: 25183255
-
Integrated transcriptome and metabolome analyses provide molecular insights into the transition of flower color in the rose cultivar 'Juicy Terrazza'.BMC Plant Biol. 2025 Jul 4;25(1):883. doi: 10.1186/s12870-025-06794-2. BMC Plant Biol. 2025. PMID: 40615831 Free PMC article.
-
Signalling cascades choreographing petal cell death: implications for postharvest quality.Plant Mol Biol. 2024 May 28;114(3):63. doi: 10.1007/s11103-024-01449-6. Plant Mol Biol. 2024. PMID: 38805152 Review.
-
Methylation Modification in Ornamental Plants: Impact on Floral Aroma and Color.Int J Mol Sci. 2024 Jul 29;25(15):8267. doi: 10.3390/ijms25158267. Int J Mol Sci. 2024. PMID: 39125834 Free PMC article. Review.
Cited by
-
Differences in the Abundance of Auxin Homeostasis Proteins Suggest Their Central Roles for In Vitro Tissue Differentiation in Coffea arabica.Plants (Basel). 2021 Nov 27;10(12):2607. doi: 10.3390/plants10122607. Plants (Basel). 2021. PMID: 34961078 Free PMC article.
-
Widely targeted metabolomics reveals the antioxidant and anticancer activities of different colors of Dianthus caryophyllus.Front Nutr. 2023 May 19;10:1166375. doi: 10.3389/fnut.2023.1166375. eCollection 2023. Front Nutr. 2023. PMID: 37275648 Free PMC article.
-
Transcriptomic analysis unravels the molecular response of Lonicera japonica leaves to chilling stress.Front Plant Sci. 2022 Dec 22;13:1092857. doi: 10.3389/fpls.2022.1092857. eCollection 2022. Front Plant Sci. 2022. PMID: 36618608 Free PMC article.
-
Analyzing Morphology, Metabolomics, and Transcriptomics Offers Invaluable Insights into the Mechanisms of Pigment Accumulation in the Diverse-Colored Labellum Tissues of Alpinia.Plants (Basel). 2023 Nov 3;12(21):3766. doi: 10.3390/plants12213766. Plants (Basel). 2023. PMID: 37960122 Free PMC article.
-
Comparative study on the mechanism of yellow petal formation in Paphiopedilum armeniacum: an integrated transcriptomic and metabolomic analysis of three Paphiopedilum species.BMC Genomics. 2025 Jun 4;26(1):560. doi: 10.1186/s12864-025-11648-0. BMC Genomics. 2025. PMID: 40468222 Free PMC article.
References
-
- Strauss SY, Whittall JB. Non-pollinator agents of selection on floral traits. In: Harder LD, Barrett SCH, editors. Ecology and Evolution of Flowers. Oxford: Oxford University Press; 2006. pp. 120–38.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

